In 8 years, we’ll be 15 million healthcare workers short. And even with access to healthcare, patients ignore at least 20-30% of doctors’ prescriptions. Not to mention that the number of patients with chronic cardiovascular and chronic respiratory diseases, and diabetes will only grow in the next decade. These are the three major reasons we’re seeing rapid growth in digital therapeutics. No, not “digital health” but “digital therapeutics (DTx)” (I’ll explain the difference later). The industry is now worth $6.5 billion with a %31.5 between 2023-2032 CARG growth is revolutionizing the way we treat chronic diseases. In 2021, the number of people using DTx solutions doubled since 2020 reaching 44 million users (source) .
There are only 8 unicorn digital therapeutics startups and hundreds more entering the competition. And they all have two problems – lack of tech experts for building digital therapeutics solutions and security and privacy issues.
What are digital therapeutics?
Digital Therapeutics Alliance’s definition of digital therapeutics interprets DTx as an application of clinically evaluated and evidence-based software to prevent, manage, and treat a wide range of behavioral, mental, and physical illnesses and disorders.
EDPS definition of digital therapeutics categorizes DTx solutions as “evidence-based therapeutic interventions driven by software to prevent, manage, or treat a medical disorder or disease (source). In other words, DTx are patient-facing software applications that help patients treat, prevent, or manage the disease and that have a proven clinical benefit."
FDA categorizes DTx solutions as Software as a Medical Device which means that it has to be prescribed like pills. And just like pills, DTx solutions have a direct impact on the health of people.
The goal of DTx solutions, a subset of software as a medical device (SaMD), is to conduct interventions using software that utilizes artificial intelligence, machine learning, natural language processing, and other kinds of high-tech.
Digital therapeutics vs SaMD: Is there any difference between digital healthcare and digital therapeutic products?
Yes, there is. Lt's resolve a digital therapeutics vs SaMD dispute for good. Many applications and gadgets promise to enhance health outcomes but DTx solutions are supported by scientific evidence, aim for important KPIs, and typically face FDA or another healthcare body regulations.
By the way, in one of our previous articles we talked about the SaMD development process , so you can learn more about it by reading our guide.
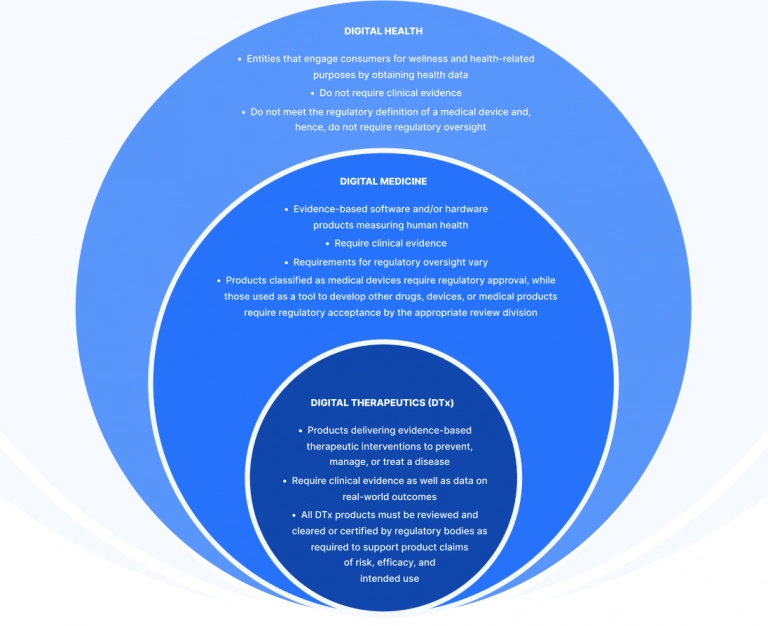
Difference between DTx and digital healthcare
Currently, the DTx solutions are successfully used for treating patients with:
- Heart diseases
- Cancer
- Diabetes
- Obesity
Build compliant and secure DTx solution with us
DTx market: Stats and facts for 2023
What’s happening with the digital therapeutics market in 2023? Remember the 44 million active DTx solutions users in 2021? According to Statista’s digital therapeutics data, in 2023 there will be 177.8 million patients whose interventions will be conducted through digital therapeutics products.
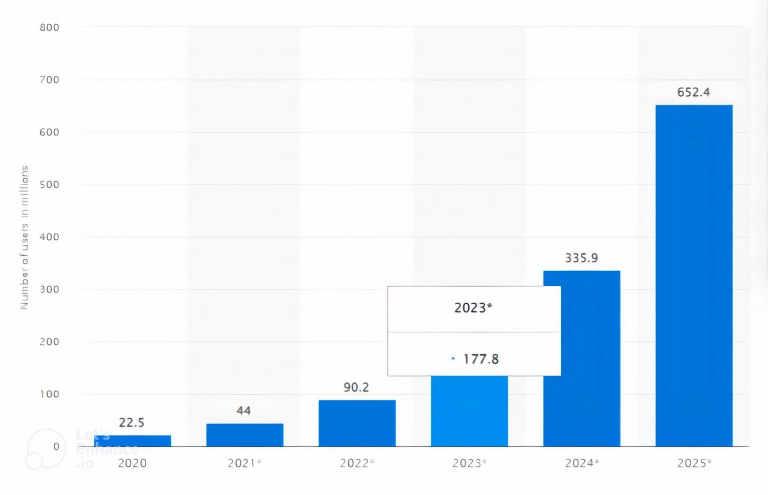
Number of people using digital therapeutics worldwide from 2020 to 2025
Another resource, the GMI gives the following data about the digital therapeutics market (source) :
- The digital therapeutics market is expected to grow by 31.5% CARG (2023-2032)
- The software segment of the market share comprised 90% 0f the global digital therapeutics market
- About 69% of the digital therapeutics market is comprised of B2B segment
- The majority of digital therapeutics vendors gain income due to a streamlined supply chain in the in B2B channel
- Asia Pacific market value reached $825 million becoming one of the largest digital therapeutics markets in the world
- Building digital therapeutics solutions remain the major growth strategy for digital therapeutics vendors
To meet the healthcare needs of the growing number of DTx solution users, countries start developing reimbursement initiatives. In light of the governmental initiatives the and spread of DTx solutions, the global digital therapeutics market has seen the following growth:
- 2020 - $2.3 billion
- 2021 - $4.20 billion
- 2022 - $5.09 billion
- 2023 - $6.93 billion
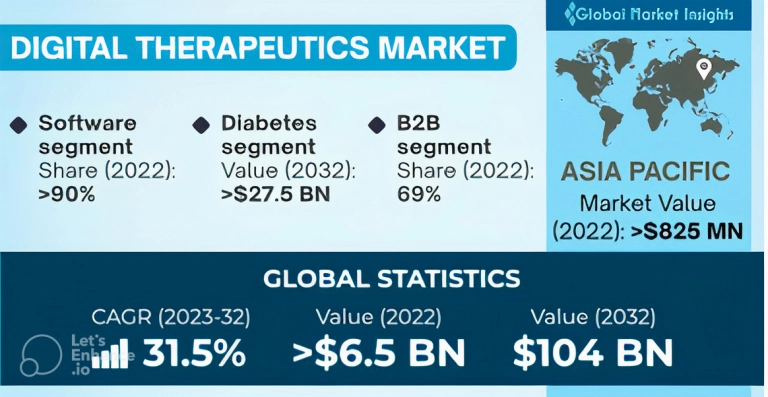
GMI's digital therapeutics data
At the moment, digital therapeutics is developing in many directions. We see so many exciting examples of digital therapeutics in healthcare that it’s hard to predict what’s next. Founders of the largest digital therapeutics products agree upon the fact that we’ll be seeing more and more interoperability, mergers, and integrations than ever before.
In an interview for McKinsey, CEO of Sonormed, one of the digital therapeutics examples of unicorns, Jörg Land says that “We will see digital therapeutics and digital diagnostics integrate into the health system and, in many cases, also reimbursed—providing a kind of guidance for the patient and also the physicians. A solution reimbursed by insurance signifies it complies with the rules of market access and that someone has had an in-depth look at the solution’s quality.”
The GVR claims that the US digital therapeutics market “is estimated to expand at a compound annual growth rate (CAGR) of 24.8% from 2022 to 2030.” With DTx solutions for diabetes and obesity treatment being the largest product group.
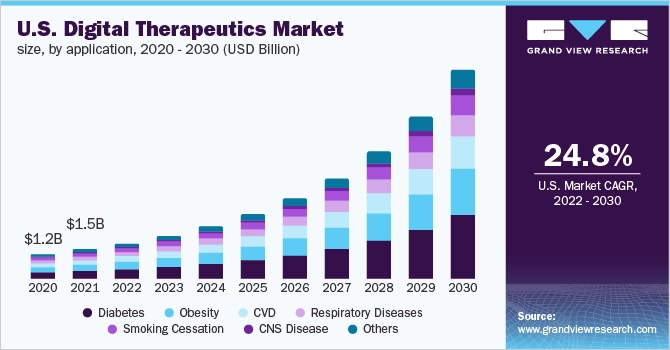
US Digital Therapeutics Market source
Planning to engineer or expand your DTx product?
Learn how to Meet FDA, DiGA, and MDR requirements

Core principles of digital therapeutics
So what does it take to have your software fit into the “DTx solutions” category? There’s actually a neat list of foundational principles the digital therapeutic products have to match. These are the most important ones listed by Digital Therapeutics Alliance source :
- Aim to prevent, manage, or treat a disease or a medical condition
- Provide medical intervention powered by software
- Adhere to design, manufacturing, and quality standards
- Engage end users while building digital therapeutics solutions and running usability testing process
- Implemthe patient privacy and security measures
- Follow best practices for deployment, management, and maintenance
- Get your product reviewed and cleared or certified by regulatory bodies to support product claims of risk, efficacy, and intended use
- Publish trial results, including clinically-relevant outcomes, in peer-reviewed journals
- Only make claims that are appropriate for the level of clinical evaluation and regulatory status
- Collect, analyze, and apply real-world evidence or product performance data
DTx product best practices
Now that we know the core principles of digital therapeutics, how do we meet them? There are a number of best practices that can help excel in DTx product design, evaluation, and deployment best practices.
Design, quality, manufacturing best practices
- Engineer software according to the ISO 14971 , IEEE P2675 , IEC 62304 , IEC 62366-1 , AAMI TIR 45
- Make sure that the product follows data-driven intervention pathways based on clinical mechanisms of acition
- Follow the Current Good Manufacturing Practices (cGMPs) including ISO 13485 , 21 CFR 820, and other relevant standards
- Implement transparent auditable validation methods
End users' engagement in product development best practices
- Aspire to meet the user’s core needs by putting the target population at the center of your product. Consider the abilities, device interfaces, environments of use, etc.
- Add universally designed features when necessary (e.g., Xcertia Guideline U9)
Patient privacy and security protection
- Follow and comply with applicable ePHI regulations
- Adhere to the GDPR and HIPAA guidelines
- Inform your users about the way your product will collect, use, and retain their data
- Safeguard the user data with proper authentication and encryption SOC 2 and HITRUST certifications
- Scan, prevent, and proactively address cybersecurity issues by actively engaging peers and stakeholders
- In case of a breach, inform the appropriate supervisory authority, and explain what needs to be done to fix the issue and reduce risk in the future.
Planning to improve interoperability?
Learn how to use FHIR standard: Value and approaches explained

Product deployment, management, and maintenance
- Create products that resolve real issues for users
- Ensure scalability of the product in all environments
- Add risk analyses and mitigation efforts in the product lifecycle
There are numerous more best practices for building digital therapeutics solutions. Digital Therapeutics Alliance updates a list on regular basis.
Read also: Digital Biomarkers in Healthcare and Clinical Trials
What are the benefits of digital therapeutics?
Digital therapeutics and their impact on healthcare can’t be overestimated. But what does it bring to the very digital therapeutics vendors and clinics?
The major benefit for digital therapeutics vendors is the ability to earn from B2B channels by integrating their solutions into healthcare ecosystems.
Especially when the products are approved by FDA, the vendors can:
- Enter new markets. By complying with local regulations issued by healthcare bodies, DTx digital therapeutic products can gain larger shares of the global market.
- Get reimbursement. Following Germany's lead, a few other European nations, notably France, Sweden, Denmark, Ireland, Finland, and Estonia, have begun implementing a standardized compensation structure. Additionally, private insurance in the US provides several choices for payment for DTx applications with UDI codes.
- Integrate with other products. The less classical way of monetizing on the DTx solution white-labeling it and integrating it into a client’s healthcare ecosystem. However, the majority of businesses follow a more traditional approach a simply integrate their products into various ecosystems, DTx products, and digital healthcare solutions.
Patients now have access to tried-and-true medical innovations that are simple to use and enhance health outcomes. They also receive individualized care and treatment plans, which are significantly more successful due to tight adherence to the therapy schedule.
Clinicians save information that may be used to further modify the patient management plan and track the efficacy of their treatment regimens.
By remotely communicating with patients with chronic diseases, healthcare practitioners may monitor patients in real-time, take prompt action, increase the effectiveness of healthcare delivery, and decrease the need for in-person visits.
Payers reduced both their out-of-pocket and premium payments for insurance.
What are the major benefits of DTx solutions? According to IBM research, the major pros are:
- 63 % individualized therapy
- 61% greater patient engagement
- 56% improved outcomes
- 43% reduced cost of care
- 30% new medical insights
- 26% better reach of care
Real use cases of DTx implementation
The real-life use cases of DTx solutions come in many forms: from the ones that remind you to take a pill and log in symptoms to the ones that connect patients with therapists.
Propeller is one of the cutting-edge examples of digital therapeutics that operates with chronic condition management DTx solution used for the management of asthma diabetics. The platform has proven to increase medical adherence by up to 58% and reduce healthcare visits relate to COPD issues and hospitalization by 57% and 35%, respectively.
Kaia Health’s solution is one of the virtual coach DTx solutions. The application shows physical exercises and uses computer vision in order to provide feedback to patients with MSK conditions. It also increases the interaction rate by using avatars and integrated voicing.
Approach to creating digital therapeutics solutions
There are two approaches to building digital therapeutics solutions: doing it in-house or delegating the engineering part to a tech company with experience of creating digital therapeutic products.
In-house DTx solution development necessitates a cross-functional in-house team comprised of:
- Regulation experts (sometimes those are business analysts)
- Clinicians with expertise in a particular area of treatment your app covers
- Software engineers, quality assurance specialists, and DevOps engineers
- Product manager
- Data scientists (in case you want to use AI and ML)
In case you have all those specialists on board, most likely, you will follow a standard DTx solution engineering path.
1. Deciding on the type of DTx solution
A digital use case scenario based around the idea of using a certain digital capability to remedy a disease or condition. Example: You decide to create a DTx solution that makes use of computer vision technology to control the way patients do physical exercises. The scenario is encouraging and transformative, but it can also be more challenging to implement because it calls for more commitment and more money.
The therapeutic area scenario predicts that DTx product development is decided by MedTech and pharmaceutical businesses based on their clinical and therapeutic competence. They select a certain therapeutic field that needs novel strategies to enhance or replace current therapies.
Read Also: Digital Therapeutics and Pharma
2. Creating a pre-clinical MVP
After you’ve crystallized the vision of how the DTx solution will resolve a real medical problem, you can move to the engineering part.
At Binariks (that’s our company, we wrote this article with our specialists), we follow a four-fold engineering process:
- Gather requirements
- Conduct solution design
- Engineer DTx solution
- Run code quality check
- Deploy the product
After a pilot version of the application gets clearance, it’s ready to undergo more clinical trials and prove practical value to healthcare authorities.
How can Binariks help?
We offer a wide range of DTx solution engineering services including full-cycle development, solution architecture adjustment, solution design, compliance consulting, and many others.
- Scale and integrate your DTx product
With Binariks, you can expand the product functionality while getting clearance from healthcare bodies faster and less painfully.
Our tech experts will help you reach interoperability and integrate your product with other healthcare ecosystems. We have profound expertise in creating interoperable solutions with HIPAA-compliant architecture .
With us, you may scale product functionality while obtaining approval from healthcare bodies more quickly and painlessly.
Our engineers will help integrate your solution with various and reach interoperability. We are highly skilled in developing systems that are interoperable and have HIPAA-compliant architecture.
- Make a DTx solution design
We have a solution design team comprised of solution architect, business analyst, and engineers. They specialize in creating solutions that inherently follow the HIPAA rule and utilize compliant technologies. They will also help you add functionality to your existing DTx solution that require MDR or FDA approval.
Here’s how a solution design process at Binariks looks:
- Understanding the problem to be solved
- Analyzing the users
- Working out the vision statement of the DTx
- Validating DTx idea (going through 3 stages of validating the solution: acceptability, feasibility, effectiveness)
- Learning the implementation regulations
- Defining the requirements and scope
- Consult you on DTx solution compliance and regulations
It’s not easy to pass clearance on the first attempt. Your specialists help businesses find clarity and create actionable plans for building compliant DTx solutions. We help create digital therapeutic products that comply with the HIPAA, HITECH, FDA, and other important regulations.
- DTx product engineering
Binariks follows product security and compliance practices at every stage of the DTx SDLC. We run automation and penetration testing and follow Scrum ceremonies that predict regular discussions of product security.
Read Also: Best Practices for Secure SDLC
DTx products delivered by Binariks
Among many other healthcare products, we’ve helped numerous businesses create digital therapeutics solutions. The most recent digital therapeutics example we’ve delivered are purely DTx solutions:
YourCoach
In-app experience development for a DTx application
YourCoach is one of bringing digital therapeutics examples. The company owns a comprehensive practice management platform as well as an operational environment that facilitates cooperation between health and wellness coaches, their customers, businesses, and workers. YourCoach enhanced the integration of its HIPAA-compliant platform with its client's DTx products by engaging our engineers.
Today, YourCoach is considered one of the most advanced digital therapeutics companies in the healthcare sector. Therefore, we are very proud that we were able to help them develop such a solution.
Medloop
Architecture consulting for an integrated patient management tool
Client's digital therapeutics product offers evidence-based preventative medicine to improve illness outcomes, chronic disease incidence, and care management. Our team examined the product architecture, connected the system with the UK NHS, and assisted with HIPAA compliance.
Final thoughts
Between 2023 and 2032, the digital therapeutics sector is expected to develop at a compound annual growth rate of 31.5%. This expansion is being driven by a dearth of healthcare staff, people who do not follow physicians' orders, and a rise in chronic illnesses. The sector is now worth $6.5 billion, and the number of users is expected to quadruple in the following years. Despite hurdles such as a lack of technical competence and security and privacy concerns, many new firms are entering the sector, which is likely to stimulate innovation and growth in digital therapies.
Contact us if you’d like to talk about your DTx solution.
FAQ
Share

