Сlinical trial is an essential part of the development of new treatments or medical devices. It is a responsible, complex, and expensive study, generating large amounts of valuable data. The accuracy and completeness of the data collected directly impact the safety and efficacy of the products being studied.
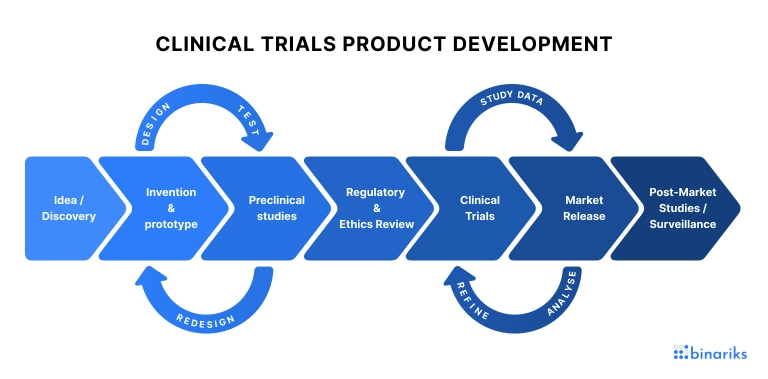
No wonder, pharmaceutical and medical device companies are always interested in innovative ways to optimize data management activities. And electronic case report form software is the answer.
This digital alternative to the paper-based case report forms is used in clinical investigations to collect data on trial participants, treatment, outcomes, etc., and immediately enter them into the database. It also streamlines the process of analyzing clinical data and improves its quality while saving costs.
Keep reading and learn more about the benefits of switching from paper to electronic CRF.
Advantages of eCRFs
Electronic Case Report Forms (eCRFs) offer numerous advantages over traditional paper-based methods in clinical research. For example:
- Improved data accuracy and completeness: Electronic CRFs provide pre-validation and standardization of data. They have in-built validation rules, such as edit checks, which prevent data entry errors or flag potential them for correction.
- Real-time data entry and access: Electronic CRFs allow for real-time data entry, leading to faster decision-making and enhancing collaboration among study team members. Authorized users can access data over the internet, wherever they are.
- Improved data security and privacy: Electronic data can be encrypted, password-protected, and stored in secure data centers. Electronic CRFs allow for more granular control over user access, ensuring that only authorized individuals can view and modify data.
- Streamlined data management and analysis: Electronic CRFs automate data entry, validation, and cleaning, reducing the time and resources required for manual data entry and cleaning. And with the ability to integrate with other data sources, like electronic health records (EHRs), eCRFs simplify data management and analysis.
- Faster study startup and data processing: Electronic CRFs simplify and streamline data collection, management, and analysis: This accelerates the research process, allowing for more efficient and effective clinical trials and other studies. By automating the data entry process, сlinical research assistants and principal investigators can begin the data analysis process sooner.
And finally, speaking of the advantages of an electronic case report form, it's worth mentioning how their implementation benefits not only clinical data managers and investigators but medical device manufacturers.
According to the study comparing the efficiency of electronic and paper case report forms, electronic CRFs help significantly reduce data collection costs. The total cost per patient with electronic forms was more than three times lower than that with paper (source ).
Besides, with reusable edit checks and custom setups of electronic CRFs, study-building time can be reduced by up to 41% compared to paper (source ).
COVID-19 Testing Mobile App
We've revamped an FDA-authorized self-testing outbreak monitoring app, read how
Key features of eCRF systems
Here are the most valuable features of electronic CRFs facilitating the daily work in medical device clinical trials:
User-friendly interface for efficient data entry
Optimal eCRF design and user-friendly interface facilitate easy and efficient data entry. And clear instructions help to reduce data entry errors and ensure complete and accurate data collection.
Thus, half of the surveyed investigators mentioned that they adapted to the user interface from the first patient. Two-thirds of the respondents were satisfied with the intuitiveness of the system interface (source ).
Automated data integration with other clinical trial systems
eCRF systems for medical devices can integrate with other clinical trial systems, such as electronic data capture (EDC) systems and electronic health records (EHRs).
The recent study of the potential benefits of automated EHR-to-CRF data transfer concluded that this feature helps to minimize errors while increasing the timeliness and efficiency of data management tasks (source ).
Comprehensive data tracking and audit trails
Electronic CRF systems for medical devices have robust data tracking and audit trail capabilities.
An audit trail module prepared following the 21 CFR Part 11 requirements allows tracking each field's complete change history in each form of the electronic CRF (source ). This helps to maintain transparency and accountability in data management and analysis.
Support for regulatory compliance and data standards
Using electronic CRFs can help a medical device company maintain regulatory compliance with ICH-GCP (International Conference on Harmonisation – Good Clinical Practice) guidelines and the Clinical Data Interchange Standards Consortium (CDISC) standards. (source /source ).
Adherence to ICH-GCP and CDISC standards ensures data quality and protects the rights and confidentiality of trial subjects.
Customizable forms and data validation rules
By creating custom forms and validation rules through electronic CRF systems, you can design your electronic case report forms applicable to the study requirements (including the ability to capture specific device-related data).
Customizable data validation rules ensure data accuracy and completeness, reducing the need for manual data preparation.
What regulations apply to your SaMD?
Learn whether your product has to comply with the latest regulations

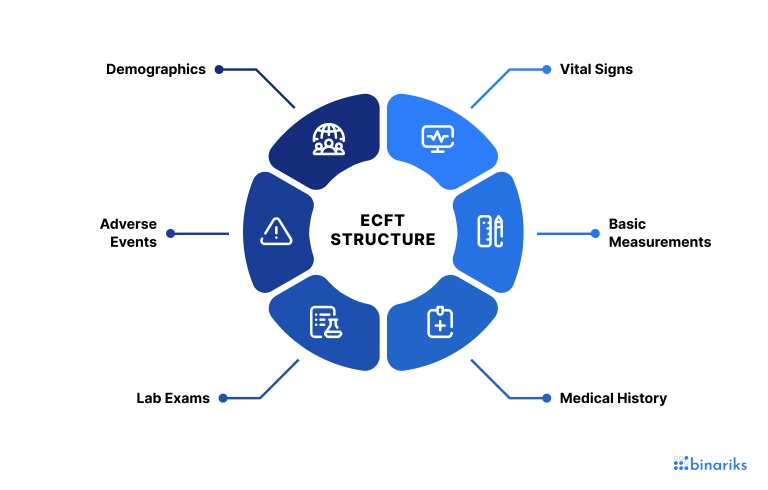
eCRF components
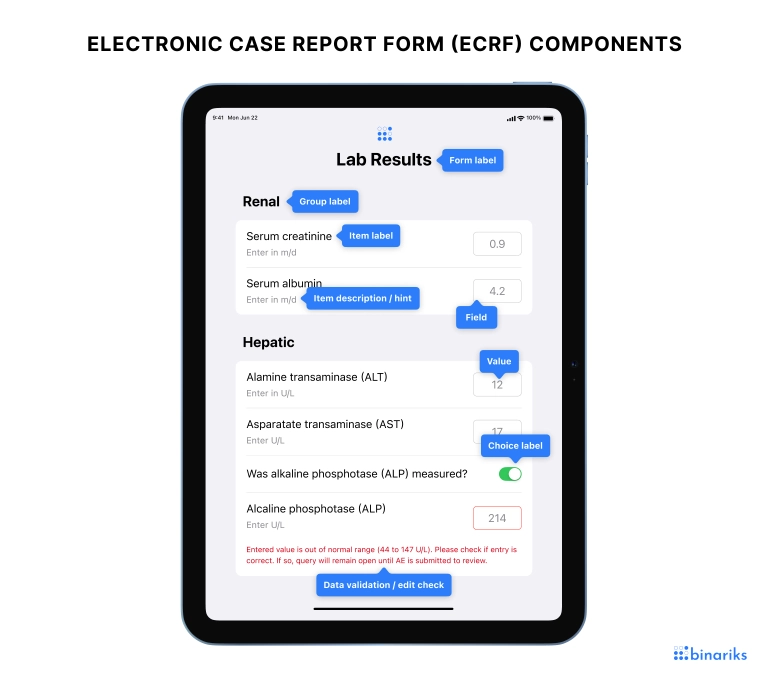
Let's take a look at the eCRF components and what function they serve.
Component | Description |
Form label | The name or title of the specific case report form |
Group label | A label used to group several related items (sub-categories) within a form |
Item label | A label used to identify data fields within a form and to prompt a user to enter data within a particular field |
Item hint | Supplementary text about the item (or placeholder text) that gives a user a hint about the data they need to provide |
Field | The area where data is entered into the form. It can be either in the form of a standard input field, or checkboxes, radio buttons, etc., but corresponding to an individual item. |
Value | The actual data entered into the field by a user or automatically generated/calculated by the form itself |
Choice label | Labels or options available to a user in a dropdown menu or radio button group |
Data validation/edit check | Automatic checks that ensure data entered into the form conforms to predefined rules and constraints, such as data type, range, or consistency with other fields |
Implementation of eCRFs
Implementing and adopting eCRFs for medical devices can be a complex process, but there are several best practices that can help achieve a successful outcome.
Here are some tips to consider:
- Engage with all stakeholders, including clinicians, researchers, regulatory experts, and IT professionals, to ensure that the eCRF meets everyone's needs and requirements.
- Ensure that the eCRF meets all regulatory requirements, such as 21 CFR Part 11, which outlines the electronic record-keeping requirements for medical devices.
- When designing electronic case report forms, make sure it is easy to use and navigate, with clear and concise instructions and labels, to ensure that users can quickly and accurately input data.
- Build in data validation and edit checks to help ensure that the data entered into the CRF is accurate and consistent.
- Provide thorough training for all users to ensure that they understand how to use the CRF and how to avoid common errors.
- Monitor data quality regularly to ensure that the CRF is producing reliable and accurate data.
- Address data security. Ensure that the CRF is designed with appropriate data security measures to protect sensitive patient data.
- Test the CRF extensively before implementation to identify and address any issues or bugs.
- Offer ongoing support to users to help troubleshoot any issues that arise and to address any questions or concerns they may have.
Implementing and adopting electronic case report forms for medical devices requires careful planning, collaboration, and attention to detail. By following these best practices, organizations can help ensure a smooth transition to electronic record-keeping and produce reliable, accurate, and high-quality data.
Primary Care Platform
We engineered a platform for patient monitoring and data management
eCRF implementation with Binariks
Some names of electronic case report form systems are Sofpromed, OpenClinica, Medrio, ClinCapture, etc. Such platforms generally provide templates for CRF design and other clinical trial management solutions.
However, if you want to digitize clinical trial data and documentation with a bespoke CRF tool tailored to your specific needs and demands, Binariks specialists can help you.
Binariks has broad expertise in custom healthcare software development . We engineer EHR interoperability solutions, telemedicine platforms, cloud-based healthcare services, and other custom software products.
Contact Binariks to create CRFs that meet regulatory requirements, ensure accurate data collection, and streamline clinical trial workload.
Bring your perfect electronic CRF system into reality
Conclusion
Electronic CRFs offer significant advantages both for medical record-keeping and clinical trial management.
Using the FDA terminology, they ensure that source data meets the ALCOA principles for data integrity. This acronym stands for Attributable, Legible, Contemporaneous, Original, and Accurate, meaning that eCRFs promote the collection of accurate and complete data while complying with regulatory requirements.
Besides, with real-time access to data, study coordinators can monitor progress, identify missing or incomplete data, and respond promptly to any faults.
Thus, electronic CRFs have nowadays become a crucial element of clinical trials. Digitizing case reports saves time and reduces costs, improving the efficiency of the study and ultimately contributing to the development of safer and more effective medical products.
FAQ
Share

