The digital therapeutics (DTx) market has come a long way since the establishment of a regulatory pathway for medical devices in 1976. In the 2020s, the number of digital therapeutics solutions growth will continue, driven by increasing demand for cost-effective, evidence-based care, a surge in chronic diseases, and fast advancements in digital health technologies. As this landscape evolves, healthcare providers, payers, and regulatory agencies will play a critical role in shaping the market's future trajectory.
In this article, we analyze the current state of the global digital therapeutics market and focus in more detail on the markets in Europe and the United States, looking into unique challenges and opportunities these markets face.
Global DTx market overview
As of 2022, the global digital therapeutic (DTx) market is valued at $6.5 billion, according to research conducted by Global Market Insight. The market is estimated to grow at anywhere between 26% and 31,5% CAGP during the forecast period until 2032.
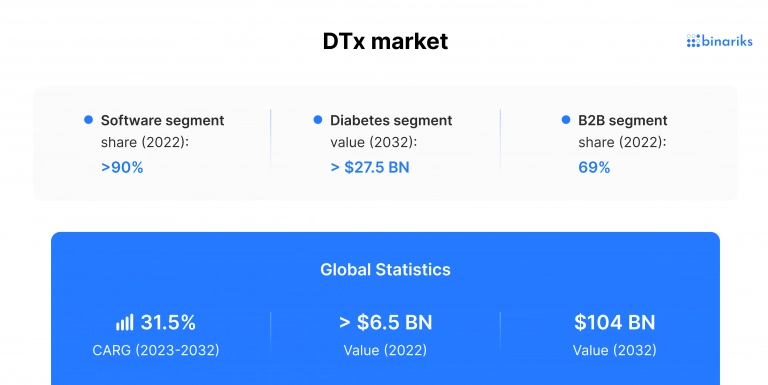
The digital therapeutic market growth is driven by various factors, including the increasing global use of smartphones, the appealing cost of digital health therapeutics applications, and the growing popularity of integrated healthcare systems. The high prevalence of chronic diseases among the aging population of developed countries also plays a significant role. Digital therapeutics growth is also fueled by venture capital, which went from $130 million in 2015 to a whopping $1.2 billion in 2019, according to the report .
Digital therapeutics market segmentation
Digital therapeutics have extensive market segmentation based on various criteria. It can be divided:
- By component, the market is divided into the software and device segments. The software segment is the largest of the two by a large margin.
- By sales channel or end-user, the market is divided into B2B (healthcare providers, pharma companies) and B2C (patients and caregivers). The patient segment is the largest among all customers.
- By application, the market fragments include diabetes, obesity, respiratory diseases, cardiovascular diseases, neurological disorders, DTx for mental health, drinking and smoking management, rehabilitation, and others. It can also be divided into preventive applications (pre-diabetes, health management, disease prevention) and treatment and rehabilitation applications.

Regions significant for digital therapeutics statistics are North America, Asia Pacific, Europe, South America, Africa, and the Middle East. Countries outside of North America and Europe worth watching for are Japan, China, India, Australia, South Korea, Brazil, Mexico, Argentina, South Africa, Saudi Arabia, UAE, Turkey, and Israel.
Become FDA/ DiGA/ MDR approved
Read a guidebook for digital healthcare actors entering the DTx field

DTx market in Europe
The digital therapeutics market in Europe is developing at a rapid pace. Only a decade ago, it was far removed from the competitive and vastly developing environment of DTx business in North America. Today, it is a striving business climate with exciting innovations. Let's dive into digital therapeutics statistics for Europe to gain a better understanding of market insights.
DTx EU market size and growth
In 2020, Market Data Forecast report estimated the Europe digital therapeutics market to be worth $612 million, which is slightly below the North American and Asia Pacific regions. The research suggests that the market will increase at a CAGR of 23.4% and reach 10,639.36 million digital therapeutics revenue by 2028.
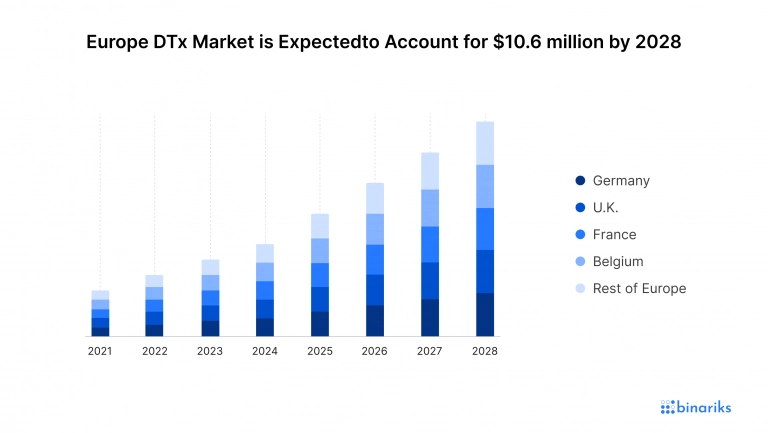
Just like in North America, the digital therapeutics market growth in Europe is motivated by factors like an aging population, growing prevalence of chronic diseases, and increasing awareness among healthcare providers and patients. Digital therapeutic solutions can help the European publicly funded healthcare system contain costs by providing cost-effective solutions with excellent potential for integration into the existing healthcare delivery systems.
Germany is the leading Europe digital therapeutics market player and a regional leader with the largest number of domestic companies. Other noticeable participants are the UK, Belgium, France, Italy, Spain, and the Netherlands. The U.K. has seen an increase in funding, which will lead to digital therapeutics market growth in the country for years to come. Steady growth is forecasted for most key European markets except for Russia.
Key players and their products
There are a variety of successful digital therapeutics companies in Europe, with many notable game-changers in Germany in particular. Here are some of the market leaders according to market research on digital health therapeutics:
Ada Health GmbH
A Berlin-based company in charge of a holistic health management app ADA, a personal health companion aimed to help with symptom assessment and quality health tracking.
Caterna Vision GmbH
A German company that provides a visual training program for children with chronic eye conditions, such as amblyopia or lazy eye.
Kaia Health
A German company creating digital therapeutic solutions for back pain, COPD, and musculoskeletal (MSK) conditions. Some well-known apps are Kaia Back Pain App and Kaia COPD App.
Medtronic Plc
An Irish DTx company with a wide range of solutions, from patient monitoring and diabetes to cardiovascular, neurological, and respiratory interventions. This is one of the largest medical devices companies in the world.
Novartis International AG
A Swiss innovative medicine company that develops schizophrenia and multiple sclerosis digital therapeutic solutions.
Sonormed GmbH
A Hamburg-based medical technology company that specializes in digital audiology. The notable app of the company is Tinnitracks, a therapeutic app for tinnitus patients.

Voluntis, Inc
A French digital therapeutic company with solutions for oncology, anticoagulation therapy, and diabetes. Applications worth paying attention to include Oleena, an app for managing the side effects of chemotherapy treatments, and Diabeo, an app with personalized insulin dosage recommendations.
Regulatory landscape in Europe
The regulatory landscape for digital therapeutics in Europe is greatly varied because it involves following regulations of the European Union (EU) and the specific requirements of each country.
For approval in the European Union, digital therapeutics solutions must comply with the Quality Management System (QMS) and Regulatory Requirements, including ISO 13485 , ISO 9001:2015 QMS, and EN 62304:2006 Software Life Cycle Processes. The required documents are endorsed by the Medical Device Coordination Group (MDCG) of the European Commission. The EU's Medical Device Regulation (MDR) came into full effect in May 2021 and is a key document for digital therapeutic regulation in the European Union.
Non-EU members also have regulations that differ from the regulations of EU members. For example, in the UK, digital therapeutics regulations are handled by the National Institute for Health and Care Excellence (NICE). The approval process is decentralized, with the final decision made by the сlinical commissioning group. The most effective regulatory landscape for digital therapeutics in Europe exists in Germany.
Regulatory landscape in Germany
As of 2022, Germany remains Europe's only country with a centralized digital therapeutics regulatory landscape. In 2019, the government passed the Digital Care Act, which creates a fast-track process for digital health apps. According to this legislation, doctors can prescribe DTx to any patient with public insurance.
The fast-track procedure that has to be followed for approval is relatively straightforward:
- Manufacturers register the CE-marked device at BfArM, Europe's largest drug approval body, submitting product functionality and comparative studies data.
- If the requirements are followed, and evidence of positive impact is present, the product is registered with DiGa.
- If the results of comparative studies are not ready, the product can receive preliminary admission to DiGa for 12-24 months.
A centralized regulatory procedure of this nature is not present in the legislature of most European countries, but some of them, like Belgium, take inspiration from Germany for updated legislation.
Primary Care Platform
We engineered a platform for patient monitoring and data management
Challenges faced by digital therapeutics in Europe
Europe's digital therapeutics market faces global challenges, as well as unique challenges that exist only in European content and stem from the region's distinctive pathway of market development. Here are some of the challenges to watch for:
- Diverse regulatory landscape: Europe has a complex and varied regulatory environment, with different rules and requirements across EU member states. Navigating these diverse regulations can be time-consuming for digital therapeutics companies operating in one country and the EU market. A relative lack of standardization is a problem that governments are just starting to address.
- Data privacy and security: The European Union is known for strict data privacy regulations, such as the General Data Protection Regulation (GDPR). Digital therapeutics companies must comply with GDPR and other relevant rules while protecting sensitive patient data.
- Integration with healthcare systems: Healthcare systems in Europe vary, so the integration process with multiple systems can become a challenge. In general, interoperability and data sharing are the keys to smooth and successful integration.
Digital Therapeutics (DTx) Explained: All You Need to Know
DTx market in the US
The US digital therapeutics market is the unquestioned leader among all regions where DTx technologies strive because of the favorable regulatory environment, exceptional competition, and business environment where new technologies emerge all the time. Let's examine the market that started it all in more detail.
The US DTx market size and growth
The US digital therapeutics market is valued at $ 2.05 billion in 2022. By 2030, it is expected to reach $ 12.09 billion, with an anticipated annual growth of 24.85%, according to Precedence Research. North America accounts for over 40% of the global digital therapeutics market, and the majority of the digital therapeutic companies in the region are located in the United States.
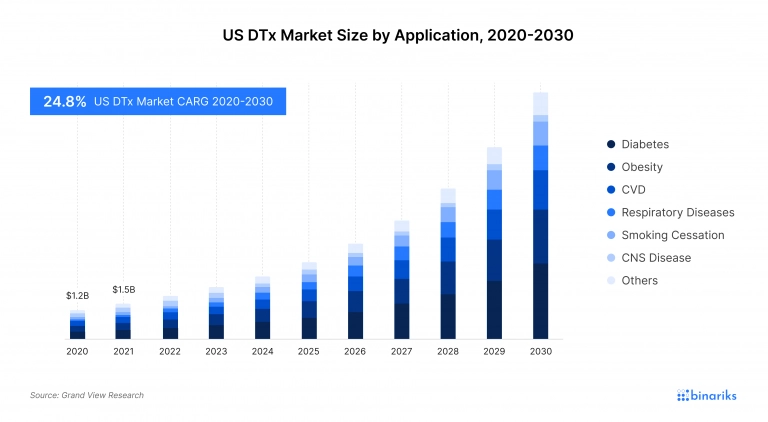
The country has the largest digital therapeutics market size and best SaMD companies because of several factors, including solid market demand, high healthcare expenditure that results in a well-funded market, mature venture capital ecosystem that leads to a favorable investment landscape, proximity of research institutions, and the presence of a clear regulatory environment.
Digital therapeutic companies can be found throughout the United States, but there are several hubs where these businesses are striving. There are California (Omada Health,Big Health), Massachusetts (Wellframe, Cogito, and Click Therapeutics), and New York (AiCure, Kaia Health). California leads the way because of the striving biotech industry in Silicon Valley. At the same time, the Greater Boston Area in Massachusetts is the home to prestigious research institutions that make biotech strive. New York is generally an environment where innovative startups strive due to the accommodation of capital.
Key players and their products
The striving digital therapeutics companies in the United States include large corporations with rich histories and startups that skyrocketed in recent years. The business landscape in the country is varied, allowing many companies to strive. Here are some of the top DTx businesses in the US:
Omada Health
A digital health company with a focus on identity management that provides cloud-native IGA solutions for enterprises.
Big Health
A company headquartered in San Francisco known for developing apps for mental health. Their key products are Sleepio, an app for insomnia management, and Daylight, an app for anxiety management. Both apps use elements of cognitive behavioral therapy.
Propeller Health (ResMed)
A California-based company focusing on solutions for sleep apnea and respiratory diseases. The company's main product is a sensor-based system that connects to inhalers used by patients with these conditions.
Fitbit LLC
A consumer electronic company specializing in wearable technology like physical fitness monitors and activity trackers. Key products are fitness trackers, smart watches, smart scales, and Fitbit App, which integrates all these products into one interface.
Welldoc, Inc.
A Maryland-based biotechnology company specializing in AI-driven digital coaching solutions for chronic diseases like diabetes. The main product is BlueStar, the app for managing diabetes.
Canary Health
A Californian company specializing in health management programs for arthritis, heart disease, diabetes, and depression. A notable product is a chronic disease management app Better Choices, Better Health (BCBH).
Akili Interactive Labs, Inc.
A Boston-based prescription digital medicine company with products like EndeavorRx, an app for the attention treatment of children with ADHD. The company creates video games designed to treat various cognitive disorders.
HYGIEIA is a Michigan-based company responsible for d_Nav, an application that regulates insulin injection in diabetes patients.
Regulatory landscape in the United States
The regulatory landscape for digital therapeutics in the United States is more centralized than in Europe. It is fully overseen by the Food and Drug Administration (FDA). FDA regulates all medical devices and ensures their safety and efficacy.
Digital therapeutics are classified as medical devices under the FDA's regulatory framework. Their specific classification (Class I, II, or III) depends on the level of risk associated with their intended use.
To prove FDA compliance and enter the market, the product goes through various stages of approval. First, the product is submitted for premarket notification (510(k)) or premarket approval (PMA). Then, digital therapeutics creators must provide clinical evidence demonstrating the product's safety and efficiency. After regulatory decisions and product launch, the devices are subjected to post-market monitoring. The general FDA regulations for Design Controls 21 CFR 820.30 apply to all medical device software.
Challenges faced by digital therapeutics in the US
- Security and privacy: Personal data security is a challenge for digital therapeutics in any environment, and the United States is no exception. Companies must comply with HIPAA regulations and maintain robust security measures to protect patient information.
- Lack of skilled professionals: The market of digital therapeutics in the United States is growing so steadily that the available pool of professionals is only sometimes enough to sustain it. Some factors contributing to the shortage of professionals include the interdisciplinary nature of digital therapeutics, evolving technology, and the need to navigate the complex regulatory landscape. An overall shortage of healthcare professionals also contributes to the issue.
- Reimbursement and pricing: Gaining reimbursement from insurance providers, including Medicare and Medicaid, is essential for digital therapeutics to become accessible to the general population. This challenge has additional aspects in the United States, where insurance systems are complex and subjected to market change based on a combination of business and political factors.
DTx market comparison: EU vs US
As demonstrated by the market research on digital health therapeutics presented in this article, digital therapeutics markets in Europe and the United States have many similarities as both are fast-growing markets driven by tech-savvy aging populations looking for smart solutions around rising healthcare costs. However, there are several factors where European and US markets differentiate.
DTx market in the US vs Europe
Market | The US | Europe |
Market Value | $2.05 billion | $612 million |
Projected CAGP | 24.85% | 23.4% |
Key Companies | Akili Interactive, Omada Health, Welldoc, Fitbit, Big Health. | Ada Health GmbH, Kaia Health, Voluntis, Medtronic |
Essential Regulations | FDA | EU regulations (MDR, GDPR MDCG) + regulations of each country |
Market maturity
The US digital therapeutics market is more mature than its European counterpart because it existed and developed for longer. There are more established corporations, partnerships, and well-known products. However, the growing European market is catching up at a full-fledged pace.
Regulatory landscape
The regulatory landscape in the USA is relatively straightforward with FDA, even though there are many factors to account for. In Europe, the regulation is more fragmented, with different rules in each country.
Healthcare infrastructure
The European Union and the United States have very different healthcare infrastructures, which has an impact on DTx technologies. The USA has a private healthcare system with several important agents, while European countries have more government involvement in a mix between private and public healthcare.
Binariks experience
Navigating the rapidly developing global digital therapeutics market comes with many pitfalls and unique challenges for every market, so choosing a well-experienced partner when developing DTx solutions is a life safer. One significant criterion when choosing a partner is ensuring they are accustomed to various aspects of DTx development.
Binariks is a partner with experience in development and Q.A., market integrations, and regulatory compliance consulting with HIPAA, HITECH, and FDA.
Binariks has experience developing solutions for top DTx providers in the USA and EU. Our finished projects include YourCoach , a HIPAA-compliant practice management platform, and Medloop , an architecture consulting for an integrated patient management tool. For complete assistance from product discovery to a finalized product entering the market, contact Binariks.
Final thoughts
The digital therapeutics market will grow at unprecedented CAGP across regions, forcing all market participants to keep up with the challenges and embrace the opportunities. As the markets are trying to make regulatory landscapes easier to navigate for all parties involved, the aging population of countries with well-developed economies is placing more sophisticated demands on the products, to which businesses are happy to respond. If you are considering entering the robust global digital therapeutics market, the timing is right, as there is no oversaturation despite the high competition, and new niches continue to emerge. However, complex challenges are given in this market, so it's best to be prepared.
FAQ
Share

