Digital therapeutics (DTx) are a well-known emerging healthcare sector, and DTx benefits are already well-documented. Nevertheless, the industry is yet to reach its full potential. One understudied aspect of DTx is its positive impact on pharma companies.
Digital therapeutics and pharma can blend technology and medicine to prevent, manage, and treat diseases with standard treatments and innovative tools. This article focuses on the current state of Dtx in the pharmaceutical industry, existing partnerships, future developments, and challenges to overcome.
DTx in pharma: expert opinions
With the growth of DTx, almost every major pharmaceutical company sees the value in appropriating DTx treatment for their use. This results in an extensive collaboration between DTx companies and big pharma. There are different business approaches to digital therapeutics in the pharmaceutical industry. Some companies are focusing on standalone DTx. And others follow "around-the-pill" DTx, working in synergy with their pharmaceuticals.
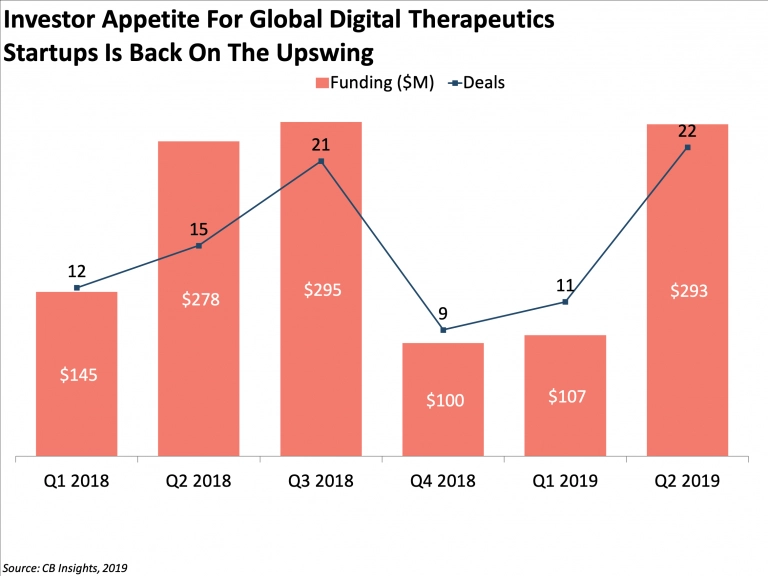
Companies like Eisai, Teijin Pharma, Sanofi, Novartis, and Bayer invest in DTx treatments for different conditions, including diabetes, cancer, major depressive disorders, and others.
"Ten years from now, I'd love to see prescribing and using digital therapeutics become just the standard of care — a normal thing, like prescribing a drug. That would reshape the practice of medicine." - Bozidar Jovicevic, head of digital therapeutics at Sanofi.
Experts across companies agree that the cooperation between digital therapeutics and pharma holds great potential. Jovicevic believes that Dtx solutions can stimulate the behavior choices of patients, which is important because negative behavior patterns lead to a significant amount of healthcare-related costs.
"It's a really exciting new area to start to think about how you can target different aspects of the same overall patient population with different digital therapeutics." - Austin Speier, a chief strategic officer at Click Therapeutics.
Other reasons why pharma adopts DTx include:
- leveraging the value of existing DTx products;
- expanding treatment options;
- embracing competitive advantage and commercial benefits of the newly emerging DTx field.
Primary Care Platform
We engineered a platform for patient monitoring and data management
The benefits of DTx for pharma
Using digital therapeutics in the pharmaceutical industry can help enhance patient outcomes. This cause and effect are demonstrated by numerous studies, even though the DTx market is still in the early stages of cooperation with pharmaceutical companies.
Improved patient outcomes through personalized interventions
Digital therapeutics in pharmacy offer patients the benefits of personalized treatments that can be adjusted to lifestyle, health status, and individual preferences. Some of the personalized solutions offered by DTx apps include tailoring dosages of medication and targeted physical exercises.
The combination of digital therapeutics and pharma leads to improvements in outcomes for various diseases, including rapid blood pressure and neurological deficits after stroke (source , source ).
Expanding treatment access and affordability
Compared to standard treatments, DTx solutions have the potential to scale quickly because of technology flexibility. This allows DTx applications to contribute to access and health equity issues.
Digital therapeutics for pharma companies can help break down geographical barriers by providing treatments that can be accessed from remote locations and different countries. This is especially helpful for patients with mobility issues. Moreover, DTx applications cost less than many drugs, expanding the reach of healthcare services to low-income populations.
Empowering patients to take control of their health
DTx applications have the benefits of a user-friendly interface, interactive features, and self-monitoring tools. These features can improve self-efficacy in patients and improve their adherence to treatment, leading to better long-term health outcomes.
Studies across conditions affirm that adherence to treatment is improved with DTx. One such study positively assesses the efficacy of self-management with DTx apps in patients with coronary heart disease (source ).
Enhancing clinical decision-making with real-time data
Another benefit of digital therapeutics is its ability to collect various data from patients in real time without any extra effort that traditional methods of diagnostics require, including their heart rate, sleep patterns, activity levels, and other information. This creates an opportunity for precise and timely interventions and improvement of treatment monitoring.
This benefit creates excellent opportunities for cooperation between digital therapeutics and pharma, as the real-time data collected by applications can help enhance traditional treatments as well.
Digital Therapeutics (DTx) Explained: All You Need to Know
The future of DTx in the pharmaceutical industry
The cooperation between DTx and the pharmaceutical industry holds great promise, as many aspects of using DTx in pharma remain underexplored. Pharma partnerships could help digital therapeutics companies expand their business, and DTx offers new potential for the treatment of different diseases. Here are some of the most exciting areas to watch for:
Integration with telemedicine and remote monitoring
Digital therapeutics and telemedicine will continue to intersect with the development of technologies. Telemedicine platforms can serve as a hub for DTx and remote patient monitoring solutions, helping patients get easier access to a wide range of these products. The solutions needed to achieve that are already being developed in the business.
Targeting mental health and well-being
Mental health is one of the fastest-growing areas of digital therapeutics, thanks to the seamless integration of cognitive behavioral therapies and other remote treatments. Treatment of anxiety and depression with DTx is well-documented, but there is demand and potential across a broad spectrum of mental health issues.
One of the potential collaborations between digital therapeutics and pharma is the treatment of schizophrenia (source ). Treatments for a broader spectrum of personality disorders should be expected within the next decade.
Collaboration with pharmaceutical companies
As technology advances, more and more pharma companies will collaborate with DTx providers to deliver holistic solutions at the intersection of DTx and pharma. This leads to growing interest in DTx among competitors in the pharmaceutical business. Some of the notable cooperations between DTx and the pharmaceutical industry to watch for include:
- Bayer and OneDrop for the treatment of diabetes and high blood pressure
- Ingelheim and Click Therapeutics for the treatment of schizophrenia-related cognitive impairments
- Sanofi and Happify Health for the treatment of patients with multiple sclerosis
Regulatory framework and reimbursement policies
The regulatory landscape will continue evolving together with digital therapeutics. Today, FDA has mechanisms for the approval and reimbursement of digital therapeutics. Clearer and more comprehensive regulations are on the way as there is a significant demand for them in the industry.
In the EU, countries like Italy and France are following in Germany's footsteps to create a more optimized regulatory system for DTx in the pharmaceutical industry.
Create groundbreaking DTx solutions with us
DTx limitations in the pharmaceutical industry
Just like any innovative field, digital therapeutics experience various limitations across industries, including challenges related to regulations, data privacy, reimbursement, and adoption.
Some of the challenges, however, are specifically related to digital therapeutics in the pharmaceutical industry. These challenges, which are closely interrelated, include:
Skepticism of pharmaceutical professionals
Pharmaceutical professionals are not that fast with accepting DTx in the pharmaceutical industry. There are many reasons for that, including concerns about the efficacy of DTx solutions and regulatory issues that come with implementing these solutions.
Other reasons for skepticism include a lack of knowledge about DTx applications, and concerns about the impact of this technology on the patient-doctor relationship. Solving skepticism requires an interdisciplinary approach that includes educating healthcare professionals about technology and addressing their most pressing concerns.
Collecting evidence that proves the efficiency of DTx in pharma
The skepticism of stakeholders becomes an even more critical challenge if we consider that there is still a need for long-term research on the effectiveness of digital therapeutics in pharmacy and its side effects.
However, much practical work is being done to bring this evidence to the public. Decentralized trials are one of the most effective ways to create enough evidence for pharma digital therapeutics. Collecting evidence is further complicated by the lack of established testing and approval processes.
Lack of comparable standards of care
The challenges of collecting evidence run into this issue: there are often no comparable standards of care with DTx in the pharmaceutical industry.
Traditional healthcare treatments and medicine have established standards that are rooted in decades of extensive clinical research. However, with DTx, these standards are lacking due to the novelty of the field, regulatory issues, and fast technological changes.
This creates a closed loop: a collection of evidence is difficult because of a lack of care standards, and the standards are lacking because they are not supported by extensive research. This requires a more effective partnership of digital therapeutic professionals with all stakeholders.
Become FDA/ DiGA/ MDR approved
Read a guidebook for digital healthcare actors entering the DTx field

Binariks expertise
When developing digital therapeutics for pharma companies, seamless integration is key. Binariks is your trusted partner when developing DTx solutions that comply with regulations and seamlessly integrate with the ecosystems of pharma companies.
We offer development and QA, healthcare system integrations, regulatory compliance, consulting, and other services across all product development stages. For pharma digital therapeutics, we pay special attention to clinical validation, regulatory compliance, scalability, and all issues related to stakeholder cooperation.
Final thoughts
As evidence suggests, DTx therapies have the potential to change what the DTx industry sells, offering patients and providers a unique combination of drugs and digital services. By embracing a more thoughtful approach to DTx, pharmaceutical companies will finance new revolutionizing solutions for even a broader range of conditions.
Companies, stakeholders, and regulators have no chance but to adapt to the booming market, which will bring economic and medical benefits to everyone involved. For success, more efforts should be put into creating clinical evidence, improving the regulatory landscape, and enhancing cooperation between stakeholders.
FAQ
Share

