Digital therapeutics (DTx) are evidence-based therapeutic interventions driven by software.
Some of these innovative solutions are standalone alternatives to pharmacological interventions. And some complement traditional treatments and devices, like DTx in diabetes, oncology, etc.
Given the growing confidence of the users, the prospects for this field are optimistic. And according to recent research, the digital therapeutics market is expected to be worth $27.50 billion by 2030 (source ).
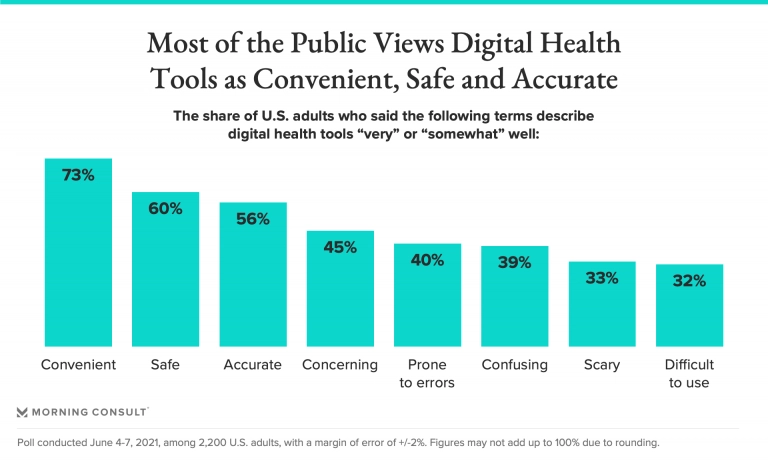
However, digital therapeutics still face some complexities and problems in terms of regulation, adoption, security, and more.
In this article, we will look at both advantages and disadvantages of DTx. We will consider six major digital therapeutics challenges, and how they can be overcome.
Regulatory challenges
One of the significant challenges facing digital therapeutics is regulatory hurdles.
All digital therapeutics must meet the requirements of the FDA, HIPAA, HITECH, or other standards depending on the region. The regulatory bodies are aimed to balance patient safety and innovation while ensuring that digital medicine is effective and reliable.
However, digital therapeutics is a relatively new part of the healthcare ecosystem, still continuing to evolve. So there can be a lack of clarity and consistency in some regulatory requirements for DTx.
This, in turn, can slow down DTx development and release into the market. Besides, lengthy legislation and approval processes complicate the entry of these technologies into new markets.
To address this problem, DTx companies can work closely with regulatory bodies early in the development process to ensure the product meets regulatory requirements.
They should also invest in rigorous testing and clinical trials to prove the safety and efficacy of their products.
Finally, to provide a more informed starting point for DTx development, companies should study existing guidelines, such as those established for medical devices or pharmaceuticals.
Become FDA/ DiGA/ MDR approved
Read a guidebook for digital healthcare actors entering the DTx field

Adoption challenges
Despite the many potential benefits of this treatment option, there are still adoption challenges faced by digital therapeutics companies.
Healthcare providers may be reluctant to adopt DTx due to their lack of awareness and skepticism toward digital health. Not only do their decisions control the lion's share of healthcare costs, but they also affect patients' lives. So no wonder healthcare providers tend to avoid additional risks in an already risky industry.
Addressing this issue requires some time and effort. DTx companies can leverage social media to build awareness and promote engagement. Advanced analytics and ML algorithms can be used to personalize the user experience and give patients unique recommendations.
Additionally, companies need to build trust with health professionals. They should provide rigorous clinical data and demonstrate the value of their products in real-world settings.
And aligning DTx with existing care pathways would make it easier to incorporate these solutions into their practice.
Working closely with healthcare providers is crucial. This allows for understanding their needs and incorporating their feedback into product improvement.
Primary Care Platform
We engineered a platform for patient monitoring and data management
Data privacy challenges
Cybersecurity is one of the major challenges of digital therapeutics relying on collecting and analyzing large amounts of personal health information.
The problem is that healthcare data is especially valuable to hackers, making it a prime target for cyber attacks (source ). Not to mention regulations like HIPAA in the US and GDPR in the EU require companies to protect patient data, further increasing the need for robust data security measures.
To protect patient data from unauthorized access, DTx companies need to implement data security protocols and encryption algorithms. They should also conduct regular security audits. This would enable timely identification ofying potential vulnerabilities and ensure compliance with applicable regulations.
Besides, companies can implement data minimization strategies or use de-identification techniques to anonymize data whenever possible. These measures are prescribed in the HIPAA Privacy Rule, covering protected health information (PHI), including data produced by DTx apps and software (source ).
Reimbursement challenges
Many insurance companies still do not cover digital therapeutics. Although being normal for all new treatments or medical devices, this can make complicate the patient's access to this treatment and slow down the company's growth.
One way to address reimbursement challenges facing digital therapeutics is to work with insurance companies and healthcare providers to establish new reimbursement policies. And another approach is to explore alternative revenue models.
Thus, companies can implement subscription-based or direct-to-consumer (D2C) pricing models. Besides, they can experiment with developing outcome-based payment. Outcome-based payment (OBP) means that reimbursement is tied to actual patient outcomes, rather than simply paying for the use of a product or service.
Finally, it's worth consistently educating payers (insurance companies, government healthcare programs, etc.) and providers on how DTx can help improve patient outcomes and reduce healthcare costs.
To sum it up, solving the reimbursement challenges of digital therapeutics requires collaboration and innovation from all stakeholders, including regulators, payers, providers, and technology companies.
Language and localization
Digital therapeutics revolutionize customized remote patient care. They provide access to evidence-based therapies from anywhere with an internet connection. That is why language and localization challenges faced by digital therapeutics companies are essential when trying to achieve cross-country scale.
DTx solutions must be tailored to specific languages, cultural contexts, and regulatory environments. Otherwise, there could be significant barriers to digital therapeutics adoption and scalability. Not to mention reputational damage to a particular company.
DTx companies can take several steps to avoid such problems. First, they should conduct thorough market research to analyze the regions where their products may be in demand. Based on this research, they can develop localized versions of their products, which would be culturally sensitive and appropriate.
It is also necessary to ensure that the DTX solution has a user-friendly interface that can be easily customized for different languages and cultural preferences. Notice that these aspects can have an impact on the therapeutic effect of the product (source ).
And, of course, companies should collaborate with local healthcare providers and regulators to ensure that their products comply with local laws and regulations.
Ongoing support
Ensuring long-term support for DTx apps also remains a significant challenge for the industry. Unlike traditional pharmaceuticals, software-based DTx solutions require continuous maintenance, updates, and technical support to ensure their effectiveness.
One of the main problems with ongoing support for DTx is the lack of trained professionals who can provide technical assistance. This is because digital health is a relatively new field.
To solve this problem, various approaches can be taken. For example, one can invest in either training and education programs or AI. Leveraging AI/ML algorithms can help create self-learning DTx solutions that could automatically identify and resolve technical issues.
Along with that, DTx companies should provide user-friendly interfaces and clear instructions and ensure that users have access to reliable ongoing support.
Primary Care Platform
We engineered a platform for patient monitoring and data management
Benefits and prospects of DTx
In 2022, $1.5 billion was invested globally in digital therapeutics, making it the fastest-growing healthtech segment (source ).
Apparently, all the mentioned challenges and disadvantages of digital therapeutics cannot outweigh its benefits. For example, such as:
- Convenience: DTx can be accessed from anywhere and anytime through mobile devices, improving care availability.
- Personalization: DTx allows for personalized treatment plans tailored to individual patient needs.
- Cost-effective: DTx eliminates the need for in-person visits and can reduce overall healthcare costs.
- Real-time data tracking: DTx allows for real-time monitoring of patient progress, which can lead to better treatment outcomes.
- Improved patient engagement: DTx can help patients become more engaged in their own care, leading to better adherence to treatment plans.
These are just some of the many benefits of digital therapeutics , and generally, the future of this field is promising.
The COVID-19 pandemic has changed the attitude of patients, prescribers, payers, and the pharmaceutical industry toward digital health solutions, including DTx (source ). And regulatory bodies have also started to recognize the importance of DTx.
For example, the FDA's Breakthrough Devices program is aimed to make it easier for digital therapeutic companies to launch their products (source ). Besides, regulatory authorities are collaborating with mature DTx startups to create structured assessment plans to remove ambiguities from the regulatory process.
While DTx challenges still exist, the market stakeholders constantly work to overcome them, adapting to the users' needs.
In terms of revenue, the DTx market was valued at $4.5 billion in 2022, and it is expected to reach $17.7 billion in 2027, growing at a CAGR of 31.6% (source ).
Final thoughts
As the demand for more personalized and convenient healthcare solutions continues to increase, DTx is poised to play a significant role in meeting this demand.
The industry evolves, and more evidence is generated every day. With all its pros and cons, DTx has the potential to become mainstream, if not as a replacement for traditional treatments, then as complementary.
Companies exploring digital therapeutics can either work in established fields where DTx has already shown positive results or explore new areas. Whatever you choose, partnering with a trusted company is essential to develop a reliable DTx solution.
The Binariks team has extensive expertise in healthtech and considers all the digital therapeutics challenges when developing a custom DTx app or software.
Contact us to translate your idea into a product!
FAQ
Share

