The software as a medical device market has grown significantly since this term was formally introduced by International Medical Device Regulators Forum in 2013.
Intensive development and a surge in new SaMD companies are caused by wide application possibilities and scalability of SaMD solutions, rising interest in SaMD using AI/ML, and in 2022, the Global Addressable market will reach $1.1 billion increasing patient demand.
So no wonder that not only separate SaMD startups offer their ideas and products. Almost all large pharmaceutical and medical technology companies seek to contribute to this niche.
In particular, they either create their own solutions, invest in SaMD companies, acquire startups and medical device companies, or collaborate with software developers with relevant expertise.
For example, with ones like Binariks. The company provides full-cycle SaMD development services and ensures regulatory compliance.
Therefore, speaking of the best SaMD companies in the US and EU, we will use the term "SaMD company" regardless of whether software as a medical device is the company's main profile.
Let's consider 14 companies that belong to key players in the SaMD market of these regions, focusing on their impact on the industry.
Top 7 SaMD companies in the US

Top SaMD Companies in the US
Digital Diagnostics Inc.
Company details
- Website: https://www.digitaldiagnostics.com/
- Headquarters: Coralville, Iowa, US
- Founder: Michael Abramoff
- Enterprise size: 51-100 employees
- Total funding: $173.4 million
Digital Diagnostics is a healthcare technology company that develops SaMD solutions for diagnosis and management.
The company's flagship product, IDx-DR, is an FDA-approved autonomous AI system for the detection of diabetic retinopathy. It analyzes retina images to identify signs of the disease in primary care settings, allowing for early intervention to prevent vision loss.
Overall, Digital Diagnostics is one of the leading players in the SaMD field, focusing on establishing automated diagnosis as the new standard of care. It should be noted that Digital Diagnostics is also among the best digital therapeutics companies .
Arterys
Company details
- Website: https://www.arterys.com/
- Headquarters: San Francisco, California, US
- Co-founder and CEO: John Axerio-Cilies
- Enterprise size: 51-200 employees
- Total funding: $71.7 million
Arterys is an internationally known medical imaging software company. It made history by receiving FDA clearance for the first-ever deep learning AI model, the Arterys Cardiac AI Model. Also, the company was the first developer of a medical imaging internet platform.
Arterys aims to transform clinical care by providing physicians with fast, precise, and actionable medical imaging insights that enable informed treatment decisions and improve patient outcomes.
For example, Arterys offers a cardiac MRI product that can precisely contour cardiac anatomy in 15-20 seconds, which would normally take 45-60 minutes to complete manually.
With FDA-cleared applications and AI, Arterys delivers customers a high return on investment with low implementation and management costs.
Viz.ai
Company details
- Website: https://www.viz.ai/
- Headquarters: San Francisco, California, US
- Co-founders and CEOs: Chris Mansi and David Golan
- Enterprise size: 201-500 employees
- Total funding: $152 million
Viz.ai is a healthcare technology company that uses AI to detect diseases and coordinate a care plan as quickly as possible. Viz.ai offers specialized AI-powered platforms across neurovascular, vascular, and cardiology diseases and other solutions used in more than 1,000 hospitals in the US and Europe.
The company's flagship product, Viz LVO, uses deep learning algorithms to analyze medical imaging and assist in the diagnosis and treatment of stroke patients. The platform enables faster identification of stroke patients, reducing time to treatment and improving patient outcomes.
And Viz.ai's newest SaMD product is Viz HCM. It is a diagnostic tool aimed to spot hypertrophic cardiomyopathy, a widespread inherited heart abnormality that can go unnoticed for a long time due to the lack of symptoms. Viz HCM is designed to aid in the identification and triage of patients who require further evaluation for this condition.
Imagen Technologies
Company details
- Website: https://imagen.ai/
- Headquarters: New York, New York, US
- Co-founder and CEO: Alex Dresner
- Enterprise size: 51-200 employees
- Total funding: $135 million
Imagen Technologies is a top provider of technology-based diagnostic imaging services. The company is committed to enhancing diagnostic imaging quality, cost-effectiveness, and accessibility.
Their FDA-cleared software (such as FractureDetect (FX), OsteoDetect, and Chest-CAD) analyzes the medical images' content, leading to better clinical diagnosis by highlighting detected pathology.
Comprehensive solutions provided by Imagen Technologies empower primary care physicians to conduct various imaging exams, including mammography, fundoscopy, and X-ray, and ensure improved access, higher accuracy, and faster results.
For example, with the help of Imagen tools, a patient can get an immediate test interpretation, a specialist final report, and a virtual specialist consult to plan further steps, all in one day.
Orthogonal
Company details
- Website: https://orthogonal.io/
- Headquarters: Chicago, Illinois, US
- Founder and CEO: Bernhard Kappe
- Enterprise size: 11-50 employees
- Annual revenue: $3.4 million
Orthogonal is a software development firm with a focus on SaMD solutions.
The company provides a full scope of services, from UX design to risk analysis, verification, and validation. They build complex SaMD and connected device systems, as well as software for Class I, Class II, Class III, and Class III implanted medical devices.
Their portfolio includes both mobile, web, and desktop apps, AI algorithms, cloud computing, and software development kits for integration to 3rd party platforms.
With a commitment to excellence, innovation, and patient-centered care, Orthogonal is helping to transform the healthcare industry and improve patients' lives.
BrightInsight
Company details
- Website: https://brightinsight.com/
- Headquarters: Sunnyvale, California, US
- Co-founder and CEO: Kal Patel:
- Enterprise size: 201-500 employees
- Total funding: $166 million
BrightInsight is a technology company that began as a SaMD startup, a mobile app to improve the treatment experience for patients with rare diseases.
Now, the company provides an Internet of Things (IoT) platform for biopharma and medtech digital health solutions. In particular, BrightInsight works with projects related to such therapeutic areas as cardiovascular conditions, diabetes, immunology, mental health, oncology, respiratory diseases, etc.
Multinational pharmaceutical and healthcare companies like Sanofi, bioMérieux, CSL Behring, etc., trust BrightInsight's expertise to enhance the value of their treatments using innovative digital tools.
Read also: Digital Therapeutics in Mental Health
Garwood Medical Devices
- Website: https://garwoodmedicaldevices.com/
- Headquarters: Buffalo, New York, US
- CEO: Wayne D. Bacon
- Enterprise size: 20+ employees
- Total funding: $14.3 million
Garwood Medical is a cutting-edge medical technology firm established in Buffalo, New York, with the goal of improving clinical results for infections and wound healing.
Garwood's products include the BioPraxTM, a minimally invasive device that uses electrodes to prevent biofilm infections on prosthetic knee implants.
In 2019, the FDA designated it as a Breakthrough Device. The business intends to begin human clinical trials in 2023.
What regulations apply to your SaMD?
Learn whether your product has to comply with the latest regulations

Top 7 SaMD companies in the EU

Top SaMD Companies in the EU
Medtronic
Company details
- Website: https://www.medtronic.com/
- Headquarters: Dublin, Ireland
- CEO: Geoffrey S. Martha
- Enterprise size: 95,000+ employees
- Total assets: $90.98 billion
Medtronic is the world's largest medical device company. Although headquartered in Ireland, it generates the majority of its profits from the US healthcare system.
Medtronic is primarily known for the development, manufacturing, and distribution of medical device-based therapies and services, but also provides SaMD solutions. These solutions are foremost designed to complement the company's medical devices and include software for remote monitoring of cardiac devices, diabetes management, and patient monitoring.
Medtronic focuses on providing products and services for the management of type I and type II diabetes, as well as for cardiac rhythm and heart failure, coronary and structural heart, aortic and peripheral vascular, spine, brain, specialty therapies, pain therapies, etc.
Overall, Medtronic technologies can improve the quality of care for more than 70 diseases.
Koninklijke Philips N.V.
Company details
- Website: https://www.philips.com/global
- Headquarters: Amsterdam, Netherlands
- CEO: Roy Jakobs
- Enterprise size: 75,000+ employees
- Total assets: $32.33 billion
Having been in the market for more than a century, Philips is a diversified technology company offering both consumer goods and professional healthcare products and services. The healthcare sector constitutes over 40% of the company's worldwide revenue and comprises three essential segments: diagnosis and treatment, connected care, and personal health.
Philips pioneered the development of digital health platforms, introducing its HealthSuite in 2014. Such platforms are aimed at expediting the development of SaMD solutions and enabling secure access to health data while adhering to regulatory and quality standards.
Through the HealthSuite platform, Philips realizes projects on medication adherence monitoring, predictive analytics, device management, enterprise telehealth solutions, etc.
Siemens Healthineers
Company details
- Website: https://www.siemens-healthineers.com/
- Headquarters: Erlangen, Germany
- CEO: Bernd Montag
- Enterprise size: 66,000+ employees
- Total assets: $49.92 billion
Siemens Healthineers is a leading medical technology company specializing in the design, development, production, and distribution of innovative diagnostic imaging systems, clinical and workflow solutions, and minimally invasive procedure systems.
The company's cutting-edge products include but are not limited to molecular imaging, ultrasound, CT, MRI, and angiography systems, X-ray products, hybrid ORs, etc.
Siemens' AI-supported diagnostics and point-of-care testing systems are used in most hospitals. These technologies help healthcare providers around the world make informed clinical decisions.
Besides, Siemens Healthineers caters to laboratories, public health agencies, universities, insurers, pharmaceutical companies, and research institutes. Siemens Healthineers is a true leader among SaMD companies creating digital therapeutics (DTx) .
MindMaze
Company details
- Website: https://mindmaze.com/
- Headquarters: Lausanne, Switzerland
- Founder and CEO: Tej Tadi
- Enterprise size: 51-200 employees
- Total funding: $340.7 million
MindMaze is specialized in neurotechnology. The company provides innovative products, including SaMD solutions and DTx, that leverage virtual reality, brain imaging, and artificial intelligence for brain repair.
For example, MindMotionGO by MindMaze is designed for patients recovering from acute inpatient settings, outpatient clinics, or home use through remote monitoring and telerehabilitation. MindMotionPRO delivers therapy to patients recovering from upper limb weakness or immobility. And MindPod Dolphin is an animated gaming experience that helps people with strokes, Alzheimer's disease, Dementia, and other neurodegenerative diseases to recover motor skills and cognitive function.
Besides, MindMaze has applied its technology in other areas, such as enhancing training regimes in sports.
Roche
Company details
- Website: https://www.roche.com/
- Headquarters: Basel, Switzerland
- CEO: Thomas Schinecker
- Enterprise size: 100,000+ employees
- Total assets: $101.3 billion
Roche is a Swiss multinational healthcare company that operates through two main segments: diagnostics and pharmaceuticals.
The company prioritizes innovation and personalized healthcare, so it is also actively developing digital health solutions.
Thus, Roche has developed a regulated SaMD dosing calculator for treating Hemophilia A. Among other examples of SaMD is a digital diabetes management system that enables patients to track their blood sugar levels and medication.
Besides, Roche provides a mobile app that helps people with multiple sclerosis monitor and manage their symptoms.
Zühlke
Company details
- Website: https://www.zuehlke.com/
- Headquarters: Zurich, Switzerland
- CEO: Fabrizio Ferrandina
- Enterprise size: 1,900+ employees
Zühlke is a global software engineering and consulting company that provides services to clients in various industries, including medical devices and healthcare. They offer expertise in digital strategy, software development, and product management.
Zühlke is known for developing innovative solutions that help improve patient outcomes and the efficiency of healthcare providers.
In particular, the company has worked on projects involving medical device development, e-health platforms, and healthcare data analytics. Some of their notable healthcare clients include Roche Diagnostics, Johnson & Johnson, and Siemens Healthineers.
S3 Connected Health
Company details
- Website: https://www.s3connectedhealth.com/
- Headquarters: Dublin, Ireland
- CEO: John O'Brien
- Enterprise size: 201-500 employees
S3 Connected Health develops and delivers wearable, implantable, and hospital-based connected medical devices and focuses on SaMD solutions and IoT.
Products created through the S3 Connected Health's Affinial platform are designed to help healthcare providers improve patient outcomes by leveraging real-time data from connected medical devices and other sources.
S3 Connected Health is partnering with pharma and medtech companies in various therapeutic areas like cardiology, neurology, respiratory, and metabolic disorders. Recent projects include the Wyss Center's Epios Cloud for personalized management of neuro-disorders, Philips Healthcare's NightBalance Lunoa device, and Cochlear Nucleus's CR120/220 Intraoperative Remote Assistant.
COVID-19 Testing Mobile App
We've revamped an FDA-authorized self-testing outbreak monitoring app, read how
Regulations for SaMD companies
The regulatory landscape for SaMD companies in the US and EU requires compliance with different regulations and guidelines to ensure the safety and effectiveness of the provided solutions. In particular:
- SaMD company in the US must submit a marketing application to FDA when it's about to release the product (source).
- SaMD company in the EU must comply with European Union Medical Device Regulation (MDR) and seek CE certification (source).
Let's consider the key differences these two systems imply:
- First, the US and EU differ in how they classify SaMD. In the EU, the term "medical device software" (MDSW) is used instead of "SaMD."
- In the US, there are three classes for devices (Class I, II, and III) based on the level of control necessary to ensure the product's safety and efficiency. MDR distinguishes four medical device classes: Class I, IIa, IIb, and III.
- The classification process also differs. In the US, an FDA database of previous devices is typically used to classify the new product. In the EU, MDSW classification requires addressing 22 rules to get a classification output (source).
Regulatory compliance is critical for SaMD companies to operate in the US and EU markets. Companies prioritizing compliance can gain a competitive advantage and strengthen their brand reputation.
According to the Fact.MR global SaMD market forecast, 2022-2023:
- 16.7% Global Market Value CARG
- in 2022, the Global Addressable market has reached $1.1 billion
- 12% historical market value growth rate 2017-2021
- North America is the largest market in 2022 followed by Europe and East Asia
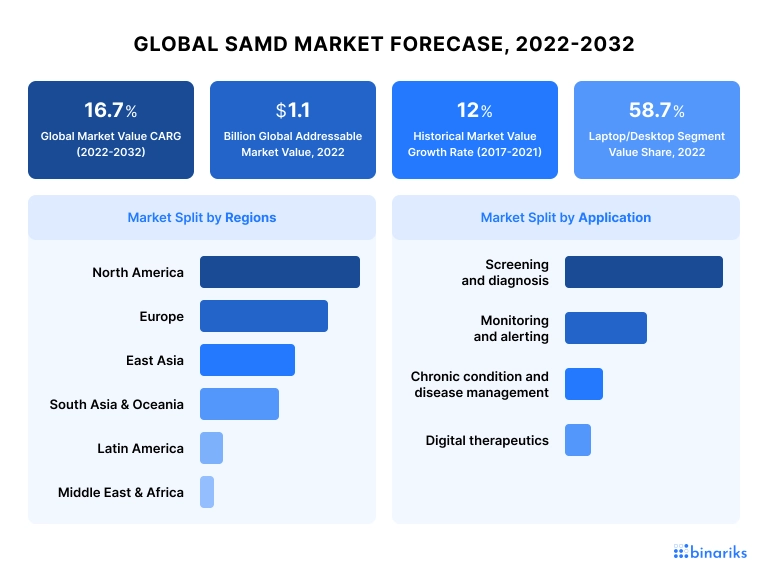
The US and EU have the largest shares of the global SAMD market
Primary Care Platform
We engineered a platform for patient monitoring and data management
Final thoughts
Even though SaMD is a relatively new tool, it has already established itself by improving the lives of millions of patients worldwide. Whether medical device startups, healthcare providers, or pharma companies, healthcare-related businesses are increasingly turning their attention to digital health solutions.
The fact is that the future of healthcare relies on software innovation, and data is the driving force behind this transformation. SaMD solutions and AI/ML are at the forefront of this shift and, according to recent market forecasts, are expected to become even more critical over the next decade.
Contact Binariks if you are also planning your next move in this promising field. We have expertise in creating scalable SaMD solutions and DTx and can provide customized, high-quality services for your project.
FAQ
Share

