Drug development is notoriously long and expensive, often taking over a decade and costing billions of dollars to bring a single drug to market. This lengthy process involves rigorous testing, clinical trials , and regulatory approvals, all designed to ensure safety and efficacy. However, the advent of real-world data in drug development offers a promising avenue to streamline these efforts.
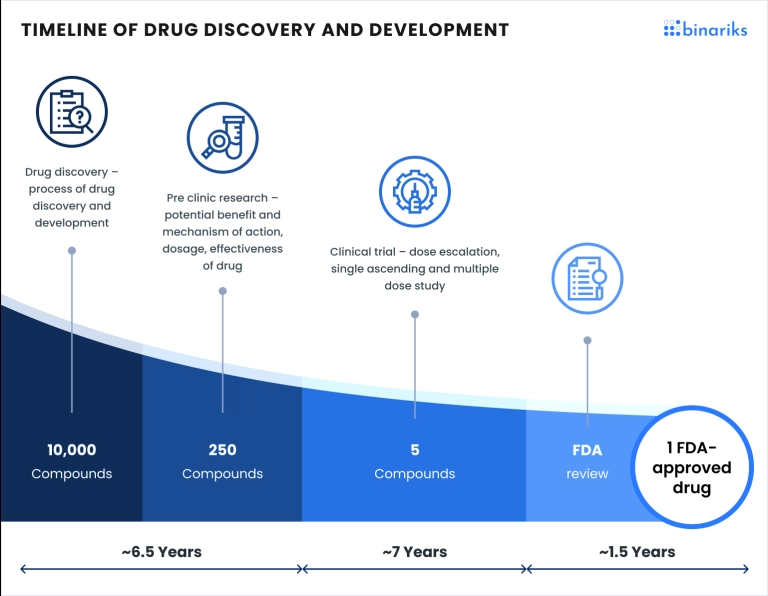
Real-world data (RWD) leverages information from everyday clinical settings, providing insights that can enhance decision-making, optimize trial designs, and predict outcomes more accurately. Integrating RWD, pharmaceutical, and digital therapeutics companies can potentially reduce the time and cost associated with drug development.
In this article, you will learn:
- How real-world data impacts drug development;
- Examples of using RWD to accelerate drug development;
- Advantages of using this data for drug development;
- Technologies that help work with real-world data;
- Challenges of using RWD for drug development;
Discover how real-world data can change drug development and offer a competitive edge.
How real-world data impacts drug development
RWD refers to health information collected outside traditional clinical trials. This includes electronic health records (EHRs), insurance claims, patient registries, and even data from wearable devices. By capturing a broad range of patient experiences, RWD provides a comprehensive view of how treatments perform in everyday settings.
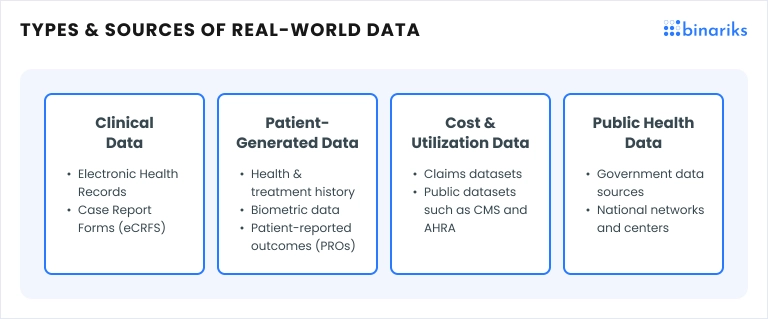
For drug developers, digital therapeutics, and pharma companies, RWD is a valuable resource that can be leveraged in various ways:
- Identify new targets for treatment: Analyzing RWD can reveal patterns and correlations that highlight potential new therapeutic targets. By understanding real-world patient outcomes, researchers can pinpoint which conditions or biomarkers are most promising for intervention.
- Evaluate the effectiveness and safety of drugs: RWD allows for ongoing monitoring of how well a drug performs and its safety profile across diverse populations. This can lead to more accurate assessments of a drug's benefits and risks beyond the controlled environment of clinical trials.
- Support regulatory decision-making: Regulatory agencies increasingly consider RWD when making decisions about drug approvals or withdrawals. By providing robust evidence from real-world settings, RWD can help demonstrate a drug's value, leading to more informed and timely regulatory actions.
Integrating real-world data into the drug development process enables pharmaceutical companies to make more informed decisions, improve patient outcomes, and expedite the delivery of new therapies to the market.
Advantages of using this data for drug development
Leveraging RWD offers numerous advantages for drug development. Here are some key benefits:
- Enhanced patient insights: RWD provides a comprehensive view of patient experiences and outcomes, leading to more personalized and effective treatments. This is a crucial aspect of real-world data analytics for drug development.
- Faster regulatory approvals: With robust evidence from real-world settings, RWD can support faster and more informed regulatory decision-making. This accelerates the approval process and ensures safe and effective drugs reach the market more quickly.
- Cost efficiency: Integrating RWD reduces the reliance on expensive clinical trials by supplementing them with real-world evidence. This approach can significantly lower the costs involved in drug development.
- Improved trial designs: By analyzing real-world data, researchers can design more efficient and relevant clinical trials. Understanding how real-world data impacts drug development allows for better-targeted studies and reduces the time and cost associated with traditional trial methods.
- Better market access strategies: Understanding the real-world effectiveness of drugs aids in developing more effective market access and pricing strategies, ensuring that treatments are accessible to a broader patient base.
Real-world data integration into drug development ultimately leads to more effective, safe, and accessible treatments for patients and provides a holistic view that traditional methods might miss.
What technologies help work with real-world data
Big data in drug discovery
Big data analytics is one of the key technologies enabling the use of RWD in drug development.
Big data involves collecting, storing, and analyzing vast amounts of health-related information, which advanced algorithms and machine learning models process to identify patterns and correlations that might not be apparent through traditional methods.
Artificial intelligence in drug development
Data scientists use statistical models, machine learning, and artificial intelligence to interpret complex datasets. This approach allows for more accurate predictions about drug efficacy and safety.
Techniques like natural language processing (NLP) can extract valuable insights from unstructured data sources like medical notes and research papers, enhancing the overall process of data science in drug development.
Cloud computing and blockchain in pharma
The implementation of RWD relies heavily on robust data infrastructure and advanced analytics platforms. Technologies such as cloud computing and distributed databases enable the efficient storage and processing of large datasets.
Blockchain technology can also ensure the integrity and security of data, providing a transparent and tamper-proof system for managing sensitive health information. Expert developers and data engineers are essential in building and maintaining these systems, ensuring the data is accessible, reliable, and ready for analysis.
Collected real-world data in pharmaceutical development is processed using sophisticated data integration tools that harmonize and standardize the information, making it suitable for analysis. Visualization tools and dashboards help researchers and decision-makers interpret the data effectively, facilitating better insights and faster decision-making.
That is why the collaboration between data scientists, software developers, and healthcare professionals is crucial for the successful implementation of RWD in drug development. Each role is integral, and together, they form a powerful team driving innovation in pharmaceutical development.
Challenges of using RWD for drug development
Despite the numerous advantages, there are several challenges associated with using RWD in drug development. Here are some key issues:
- Data quality and consistency: Ensuring the accuracy and uniformity of data used in drug development can be challenging due to the varied sources and formats of RWD.
- Data integration: Integrating RWD from different systems and sources requires sophisticated tools and methods. Big data in drug development relies on the seamless integration and harmonization of diverse datasets.
- Privacy and security: Protecting patient privacy and securing sensitive health information is paramount. Regulations like GDPR and HIPAA impose strict guidelines on handling and sharing drug development data.
- Regulatory acceptance: Gaining regulatory acceptance for RWD-based evidence can be difficult. Agencies may require additional validation and standardization to consider RWD in decision-making processes.
- Technical expertise: Implementing and analyzing RWD requires skilled professionals, including data scientists, software developers, and healthcare experts. A shortage of such talent can hinder its effective use.
- Data interpretation: Interpreting the results of RWD analyses can be complex. Researchers must carefully account for biases and confounding factors to draw reliable conclusions from drug development data.
- Infrastructure and cost: Building the necessary infrastructure to collect, store, and analyze large volumes of RWD is a costly endeavor. Companies must be prepared to invest in advanced technologies and platforms to manage drug development data effectively.
Implementing real-world data with Binariks
Integrating RWD into drug development can be complex, but with the right approach and expertise, it becomes manageable. Here are several professional tips to guide the implementation process:
- Start with a clear strategy: Define your objectives and determine how RWD will fit into your drug development process. Having a clear strategy ensures that your efforts are focused and aligned with your goals. This is crucial for successful drug development with real-world data.
- Ensure data quality and consistency: Use advanced data cleaning and preprocessing techniques to ensure your RWD is accurate and consistent. Binariks can help build robust data management systems to maintain high quality of data.
- Invest in the right technology: Implementing RWD requires sophisticated tools and platforms. We, at Binariks, can offer our expertise in integrating advanced analytics, machine learning, and cloud-based solutions tailored for real-world data pharmaceutical applications.
- Collaborate with experts: To maximize the value of RWD, engage with experienced data scientists, software developers, and healthcare professionals. With Binariks, you have access to a team of industry experts who specialize in handling and analyzing complex problems.
Binariks offers comprehensive support for integrating real-world data in pharmaceutical development. With our expertise in data management, advanced analytics, and regulatory compliance, Binariks can help your company leverage RWD and reach your business objectives.
Conclusion
RWD offers pharmaceutical companies new opportunities to gain deeper insights, design better clinical trials, and make more informed regulatory decisions for less money. Despite the challenges, the benefits of integrating RWD into drug development are clear.
To successfully implement RWD, it is crucial to have a clear strategy, ensure data quality , invest in the right technologies, and collaborate with experts. Binariks is well-equipped to support your business in this journey, creating comprehensive custom solutions and expertise to harness the full potential of real-world data.
Contact us today to learn how we can help you leverage RWD for better outcomes!
Share

