Software as a medical device (SaMD) is a type of medical software used for medical purposes that is not an integral part of a medical device. Since SaMD apps have a direct impact on health performance, they are severely regulated. Thus, creating a SaMD solution requires talents with distinctive healthcare expertise and great software engineering skills. In this article, we will shed light on the SaMD market and share the best-practices for establishing the process of custom SaMD development .
SaMD market size
Software as a medical device market is one of the fastest-growing sectors of medtech. The number of SaMD companies is growing at a faster pace compared to other types of software for medical devices. It is forecasted to reach $8.2 billion by 2027, with a projected CAGR of 11.0%.
The market is driven by increased patient demand and accelerated use of SaMD in diagnosis, treatment, and patient monitoring. AI and machine learning also contribute to SaMD market growth. Many types of software as medical device solutions are available as a part of the smartwatch experience, which drives the demand for regular customers.
Desktop/Laptop is the SaMD segment expected to dominate the market in 2027. North America occupies the highest share of the market, and this is not likely to change. Overall, investing in software as a medical device is a project not to be missed due to the growing significance of this market for different stakeholders.
Reach interoperability through FHIR integration
Streamline data exchange with integration of HIPAA-compliant standard

Who uses SaMD apps?
Various stakeholders may benefit from using SaMD and are actively interested in purchasing this type of software. Software as a medical device development requires extensive research of the target audience as each stakeholder has unique needs that inform the medical device software design. Some of the common beneficiaries of this software are:
- Medical device manufacturers
A wide variety of healthcare providers including hospitals may benefit from developing their own software as medical device apps. The common applications of SaMD for healthcare device manufacturers are pacemakers or insulin pumps, which may be interested in expanding their product offerings to use SaMD.
- Healthcare providers
A wide variety of healthcare providers including hospitals may benefit from developing their own software as medical device apps. Two simple applications of SaMD for healthcare providers qre to improve patient care or streamline administrative processes.
- Pharmaceutical companies
Pharmaceutical companies are engineering SaMD to support their drug products. For example, they may need a digital therapeutics application or remote patient monitoring systems.
- Startups
Some of the most reknown SaMD products are created and run by startups. The range of issues their SaMD solutions resolve is quite wide: from global health issues such as smoking, obesity, and diabetes to less severe including self-therapy.
Primary Care Platform
We engineered a platform for patient monitoring and data management
Core types of SaMD
Software for medical devices is a broader concept than software as a medical device. It is a computer program designed for use with medical devices in one way or another. Three custom medical device software types are SaMD, Software in a Medical Device (SiMD), and Software as an Accessory to a Medical Device.
- Software as a Medical Device (SaMD)
An independent medical software that uses information from medical devices. It can help treat diseases and perform medical procedures. It can launch independently on smartphones and other hardware that is not part of a medical device. It can also be stored in the cloud. Some prominent examples of software as a medical device include software to view the data from MRI or ultrasound examinations and apps that control medication dosage for diabetics.
- Software in a Medical Device (SiMD)
This software is part of a medical device and fulfills medical purposes but does not operate independently from the device. SiMD control and monitor the medical functions of a device are also known as embedded medical software.
- Software as an Accessory to a Medical Device
This type of software differs from other two as it does not serve a medical purpose. An example would be the software that evaluates the performance of an MRI machine and notifies the technician team when it is time to replace its components.
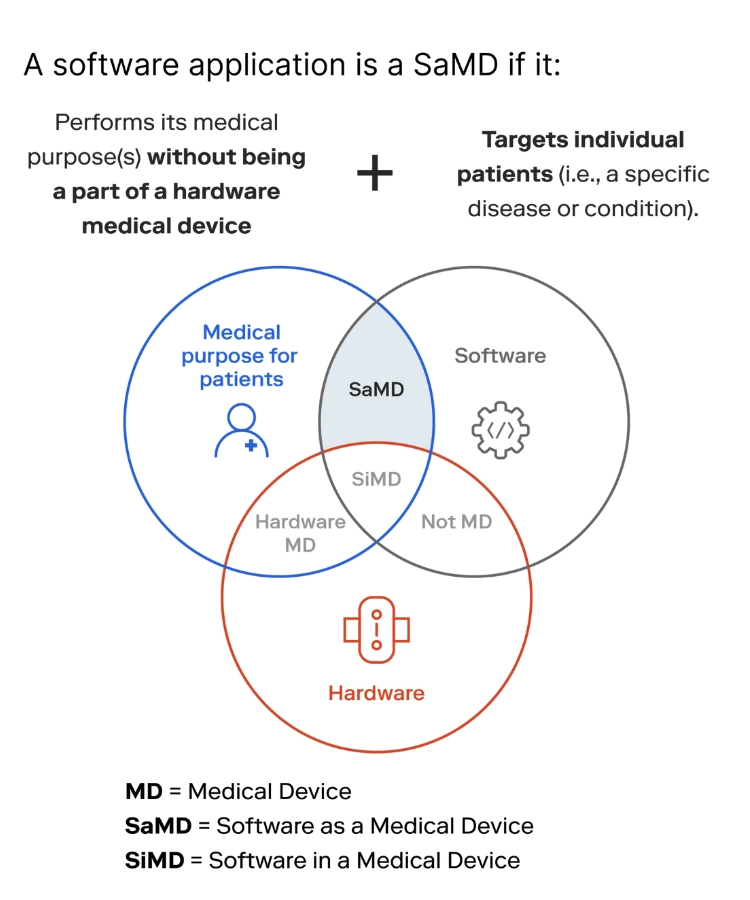
source SaMD vs SiMD vs Hardware MD
Architecture Consulting for Patient Management Tool
We audited product architecture and helped to comply with regulations.
SaMD development process
Medical device software goes through the same phases as any other type of software when in development, with the addition of regulatory approval and a more complicated validation stage. Programming medical device software follows a well-defined, structured development SaMD development process that meets specific standards and guidelines. Software development for medical devices goes through six equally important stages:
- Requirements gathering phase
The developers gather all functional and security requirements and put it into a backlog. Cybersecurity of medical devices is an important consideration at this first stage.
- Solution design phase
This stage incorporates a minimum viable product pilot run to improve user experience and the introduction of the software development plan. It also includes cooperating with stakeholders to receive background data about industry best practices.
- Development and testing phase
This is when the actual software development for medical devices takes place. Medical device software developers base their work on a prototype completed in the previous stage. Development involves coding, software testing , UI/UX design, and quality assurance. Development can be completed either in-house or with a competent partner.
- Validation and pivoting phase
Validation ensures the safety, usability, and quality of the medical device software design. This stage involves coordinating, designing, and conducting trials and research studies for product validation.
- Regulatory approval phase
Approval is a time-consuming process that involves applying to regulatory bodies, submitting the Software to them, and awaiting a reply. All previous efforts of medical device software developers are completed with this step in mind. In the USA, the product is submitted for FDA approval. In the EU, it goes through MDR approval.
- Deployment and operating phase
At this stage, the implementation of the software solution in the healthcare setting occurs. This is also when the software gets into formulary listings and when commercialization for scale is planned. When all medical device software development steps are finished, it is time for maintenance and regular updates based on customer feedback.

Programming languages
SaMD development process may involve different programming languages and additional tools. Embedded C and C++ and MatLab are used specifically for medical devices, but other popular languages are also common.
Languages for programming medical devices
- Embedded C and C++: these are the key programming languages in medical software development. The firmware for medical devices is most commonly written in embedded C because it works best for certification. It gives you the pathways to demonstrate the exact behavior of the medical device when going through the regulation process. C# is also a common choice for Software running on doctors' and nurses' computers.
- Java is a common solution for desktop software in hospitals. It is also just as popular for servers. Common medical Software developed with Java are clinical decision support systems and electronic health record (EHR) systems.
- Swift is the most common language used when developing medical device software for Apple's iOS and macOS platforms. It is a key language for everything meant for mobile.
- Python presents a viable solution for medical device programming when machine learning and artificial intelligence (AI) algorithms are involved. It is helpful for Software related to diagnostic imaging.
- MatLab is a language commonly linked to medical device coding. It has a unique image and data processing abilities and analyzes medical imaging, such as CTs and MRIs.
Tools for programming SaMD
- Compliers improve medical device coding by translating the high-level programming language code into a machine code recognized by the hardware of the medical device.
- Emulators are programs that allow the devices to imitate the functionality of another system. They are used in software development for medical devices during the testing phase for training purposes and to identify potential bugs.
- Testing software and testing devices are used in the testing stage to ensure that the custom medical device software is safe and functions correctly.
- Cloud services like AWS and Azure store information at all stages of medical software development, including collecting preliminary research, testing, and maintenance.
- Integrated health tech and wearables are becoming extremely popular among a broad category of users and serve as a gateway for more and more people to use the Software as a medical device daily.
- Front-end development tools like Node.js and React assist in building scalable projects and systems faster and improve user interfaces, respectively.
Cybersecurity for SaMD apps
Cybersecurity is a primary concern in software as a medical device development because the healthcare industry is often a subject of cybersecurity breaches. From 2009-2022, HHS' Office for Civil Rights has reported cybersecurity breaches that have resulted in a violation of 382,262,109 personal records. Health service remains one of the most vulnerable segments to data breaches.
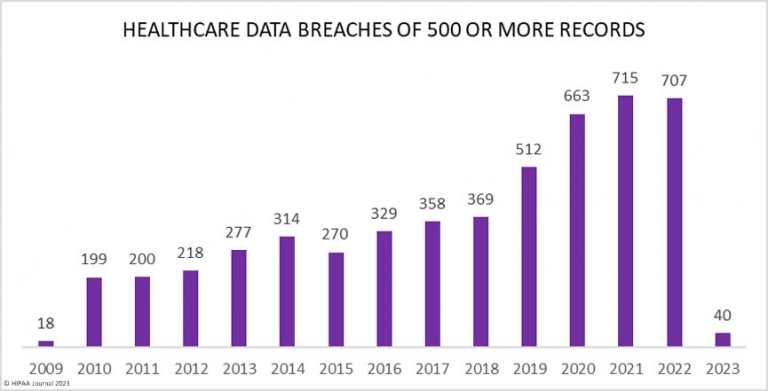
Companies working on medical device software design should pay extra attention to ensuring cybersecurity. Both the US and EU have guidelines and recommendations for cybersecurity.
SBOM and their minimum elements
Software Bill of Materials (SBOM) is a document that has to be provided by every vendor supplying their product to the US federal government. This is basically a formal inventory of software components and their hierarchical relationships. It includes proprietary and open-source code, licenses, versions, bugs, and other valuable information.
SBOM requirements are outlined in the Executive Order on Improving the Nation’s Cybersecurity . The elements that vendors have to submit with every project or technology are data fields with basic information about software components, automation support, and practices and processes needed to integrate the product into the organization.
Follow cybersecurity regulations
Regulations in the US: the FDA regulates the cybersecurity of Software as a Medical Device. The cybersecurity regulations issued by FDA are available in Draft Guidance for Industry and Food and Drug Administration Staff. This guidance supports products throughout their entire software as a medical device life cycle. It prioritizes transparency, security by design, and security risk management. There are also separate guides for devices containing Off-the-Shelf (OTS) Software and postmarket management .
Regulations in the EU: the cybersecurity of software for medical devices is regulated by MDR and IVDR Annex I. Just like the FDA document, MDR and IVDR Annex I has regulations for all steps of software development and maintenance. MDCG has useful guidance describing how to fulfill these requirements. Other useful regulations include the NIS Directive (which focuses on legal measures, and GDPR (which contains requirements to protect personal information).
Choose the right developers
The team members tasked with medical device software engineering should have experience and communication skills to bring the project to success.
However, there are also specific requirements related to SaMD development. To build custom medical device software, developers should:
- Have specific experience in software design for medical devices
In particular, developers should have a proven track record of working with the FDA or EU MDR regulations, depending on your location. They should also know how to translate medical requirements into software requirements and consider software as a medical device human factors.
- Have appropriate engineering expertise
Developers tasked with creating software as a medical device should know all programming languages required for the project, whether C++, Java, or Python. They should also have extensive knowledge of compilers, front-end development tools, and other required instruments.
- Focus on top-notch quality assurance
Quality assurance is critically important for medical device software engineering. The engineers working on software for medical devices are tasked with meeting regulatory standards. Testing should be very meticulous to ensure the safety and effectiveness of the software.
- Effortlessly communicate with stakeholders
When it comes to medical software development, effective communication is not a cliche. The team has to contact many different stakeholders all the time to ensure that time-to-market is optimal, including medical professionals and regulatory authorities.
Our experience
Binariks engineering team has vast experience creating SaMD solutions and medical device programming for various stakeholders, including tech companies, medical corporations, and healthcare providers. Our team assists clients in every step of the software as a medical device life cycle, from an initial software development plan to deployment and maintenance.
We collect medical information from third-party services and use big data analysis tools. Our teams help clients audit software architecture, comply with regulations, and accelerate time-to-market while minimizing risk and cost. We are excited to help you build custom medical device software of the future.
Final thoughts
The expected growth of the SaMD market segment creates exciting opportunities for companies and healthcare providers developing and integrating new software for medical devices into their work. In upcoming years, market growth will facilitate new technologies that will make these applications even more helpful for everyone involved.
Working with qualified experts is key when bringing new medical device software to life because of the complexity of the technology and the many regulations involved in the process. By working with an experienced partner like Binariks, you can navigate all of the complexities of medical device software design with ease.
FAQ
Share

