DTx, or digital therapeutics, is the vastly developing field at the intersection of medicine and technology that offers evidence-based therapeutic interventions for physical, mental, and behavioral conditions supported by software. To be accessible through insurance, DTx solutions should go through reimbursement just like other healthcare products do. Reimbursement for digital therapeutics is a reasonably new phenomenon. In fact, mass reimbursement was not a thing until the 2010s, and in many markets, it is yet to go mainstream, which comes with challenges and opportunities. This article explains reimbursement frameworks and ways to develop a reimbursable DTx product from the perspective of a company that offers custom DTx solutions and reimbursement assistance.
Why reimburse DTx products
Digital therapeutics reimbursement is the coverage of costs for digital therapeutics (DTx) products by either health insurance providers or public health systems. Reimbursement is the key aspect of the commercial potential of digital therapeutic applications. With reimbursement for digital therapeutics, more customers embrace DTx applications because they don't have to pay for them out of their pocket, which reduces the stakes and opens up the possibility of trying new technologies.
Reimbursing DTx solutions makes them accessible and affordable. Moreover, DTx reimbursement is the pathway to validation of product effectiveness and integration of the applications into standard care. The commercial success of DTx applications is only possible with reimbursement.
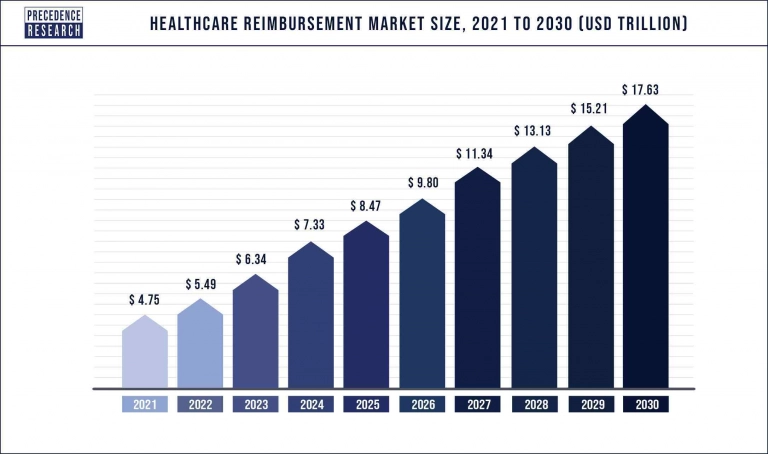
The growth of the DTx field directly depends on reimbursing DTx solutions because it provides revenue to the developing field. When DTx reimbursement policies evolved in the late 2010s and early 2020s, the DTx industry experienced accelerated growth. Likewise, DTx contributes to the growth of the healthcare reimbursement market.
Understanding reimbursement
The pathways for digital therapeutics for reimbursement vary country by country. Most common pathways involve either public or private payers. Let's look into the differences between the two.
Public health insurance reimbursement is common in countries with universal healthcare systems, such as Germany. With it, the government acts as a reimbursement agent. The applications that go through reimbursement in the country are subject to statutory health insurance.
Private insurance reimbursement is common for countries with a private healthcare system, such as the United States. In this system, private insurance companies cover DTx applications' costs. The process may involve negotiations between digital therapeutics and insurance companies.
In both public and private scenarios, the process remains quite similar. The patient visits a healthcare provider to receive a prescription for digital therapeutics. After this, the provider bills the payer for the service, and the payer covers the cost after reviewing the claim. If the coverage by the payer is not full, a co-pay from the patient may be required.
Reimbursement frameworks in the EU and US
Reimbursement frameworks for DTx products have been developed in recent years. The most sophisticated DTx frameworks exist in the United States and Germany. The latter represents the model of reimbursement framework for DTx for Europe. Below is the summary of current DTx reimbursement policies in the countries with striving DTx.
Germany
Reimbursement of a digital therapeutics app in Germany is regulated by Digital Healthcare Act – DVG. A DTx app in the country is known under digital health applications (DiGA). A reimbursement process in the country involves providing a CE marking and having privacy-GDPR compliance, as well as offering proof of requirements related to data protection, interoperability, and ease of use. Last but not least, companies are asked to submit proof of scientific evidence evaluation.
Reimbursement applications can go through the Fast-Track scheme, after which they are allowed for a limited temporary 12-month release to collect scientific proof of effectiveness. A German framework is a golden standard for other EU countries that model digital therapeutics reimbursement frameworks after the one pioneered in the country. The framework in France, similar to DiGA fast-tracking, is expected to reach the market in late 2023.
Belgium
National Institute for Health and Disability Insurance is a regulatory body that oversees the DTx reimbursement framework in Belgium. To get reimbursed, the DTx products in the country pass through the (mHealth) validation pyramid:
- M1 - CE markings and compliance with GDPR
- M2 - Evaluation for data security, confidentiality, and interoperability
- M3 - Evaluation of clinical evidence and socio-economic added value
- When evaluated by the Federal Agency for Medicines and Health Products, the apps should pass the M3 level to complete DTx reimbursement.
United Kingdom
The reimbursement pathway in the UK follows Digital Technology Assessment Criteria (DTAC). According to DTAC, applications must meet NHS standards for clinical safety, interoperability, and data protection. DTx reimbursement involves having a CE mark or UKCA mark, being compliant with GDPR, and following criteria to Digital Technology Assessment Criteria. As of 2023, there is no single digital therapeutics reimbursement network in the UK, and apps are supposed to follow the requirements of a local NHS organization.
USA
In the United States, DTx products are regulated by the FDA and labeled as class II medical devices. Medical Technology or Digital Formulary Committee reviews each new product for consideration based on FDA submission data, peer-reviewed clinical data, and HEOR data that showcases cost savings and cost offsets. Moreover, to get reimbursed for digital therapeutics , DTx products are supposed to be HIPAA- compliant. Regarding insurance coverage, private insurance provides unlimited coverage for class II medical devices and limited coverage for everything else. With public insurance, Medicaid provides limited coverage for DTx products, while Medicare does not.
Become FDA/ DiGA/ MDR approved
Read a guidebook for digital healthcare actors entering the DTx field

Real cases of reimbursable DTx products
Even though the framework for digital therapeutics reimbursement is put in place in many countries, the number of companies that received reimbursement has yet to reach its highest point. Here is an overview of digital therapeutics companies that have received reimbursement in the past few years:
EndeavorRx by Akili Interactive
Akili Interactive is a DTx company focusing on digital therapies for children with cognitive impairments. The product EndeavorRx is a video game that improves cognitive functioning in children with ADHD. The product has received FDA 510(K) approval. According to Akili's statistics , about 3% of app purchases come from reimbursement.

BlueStar by WellDoc
One of the first apps to receive traction through reimbursement, BlueStar by WellDoc is an FDA-approved DTx solution for patients with diabetes. The product received FDA clearance back in 2013 and became one of the first apps to gain certification. The application focuses on diabetes management and direct patient communication with healthcare providers. WellDoc currently gains coverage from private insurance payers, as well as a number of large major insurance companies.
Velibra by GAIA
Velibra is a German mental health app that received clearance in Germany as a DiGA under DVG. The application is meant to assist in the treatment of anxiety. It is based on cognitive behavioral therapy and offers patients an individual plan completed over six months. The DTx application is available through prescription and can be reimbursed through statutory health insurance.

Create groundbreaking DTx solutions with us
Develop a reimbursable DTx product
To get reimbursed for digital therapeutics, the DTx product has to be built with a reimbursement framework for DTx in mind. The strategy for reimbursement has to consider specific market, current and projected competition, the form of the product, and the clinical dataset
Here are the common steps and best practices that help achieve reimbursement:
- Conducting clinical trials: Digital therapeutics for reimbursement require clinical trials as they demonstrate the safety and efficacy of the product. Randomized controlled trials are common, and decentralized clinical trials are effective for DTx.
- Gathering evidence for effectiveness: DTx providers benefit from having additional channels to gather evidence. These involve real-world evidence and patient-reported outcome measures.
- Understanding regulatory requirements: Since the regulations for DTx reimbursement are complex, companies behind the product should understand them from the start. Knowing FDA or country-specific requirements in the EU helps make the right decisions about product design and clinical evidence.
- Collaborating with stakeholders and building relationships with payers: Reimbursement involves several stakeholders, including patients, healthcare providers, insurance companies, and regulatory entities. Establishing relationships with them helps to understand motivations and achieve results faster.
- Understanding the payer's perspective: Payers are a unique group of stakeholders with an unparalleled role in product reimbursement. They adopt a utilitarian approach with a focus on cost-effectiveness and clinical value. Learning to walk in a payer's shoes helps create a value proposition.
- Building a reimbursement strategy: A successful strategy involves knowledge of the landscape, gathering all evidence and data, and having a patient journey in mind. Some useful tips for building a winning strategy are to achieve positive coverage decisions as quickly as possible and start with low out-of-pocket costs for the patient.
- Developing a value proposition: A value proposition communicates why the product is worth paying for and what issues it will solve. It involves evidence-based elements like clinical outcomes, patient experience, and cost-effectiveness.
- Incorporating health economics into product development: The cost evaluation for the product should be rooted in real-world data. Cost-effectiveness analysis should consider the financial landscape and the comparable experience of other product developers.
Binariks experience
Binariks has a broad experience in creating digital therapeutic solutions. We can assist your company with digital therapeutics reimbursement in particular. Aside from developing digital therapeutic applications according to reimbursement requirements, some of the reimbursement-specific services we offer are researching regulatory compliance and creating solution architecture that complies with regulations on the chnical level. We also have a DTx solution team comprised of BAs and tech specialists which allows us to build a compelling value proposition for DTx reimbursement.
Conclusion
DTx reimbursement is key for the wider adoption of digital therapeutics and for this vastly developing sector's commercial success and reputation. The current reimbursement landscape, which varies from country to country, is complex and suffers from novelty.
However, the experience of countries like Germany and the USA demonstrates that creating understandable reimbursement frameworks is possible, and the trends show that more countries will follow. For DTx companies, cooperation with stakeholders, a well-developed reimbursement strategy, and value proposition are key for successful certification and reimbursement. Stakeholders are interested in evidence-based, cost-effective products more than in anything else.
FAQ
Share

