Sticking to outdated decision-making methods in pharmaceuticals can lead to costly delays and less effective outcomes. When research teams work in silos, critical data often gets stuck in departmental pipelines, making it hard to collaborate and keep trials on track.
The good news is data analytics in clinical trials is changing the game.
At the same time, it's primarily due to clinical data issues that pharmaceutical companies are increasingly concerned about the challenges of quickly bringing new drugs to market. A recent global study by Oracle Health Sciences and Pharma Intelligence found that 57% of clinical researchers believe these data challenges are directly causing trial delays.
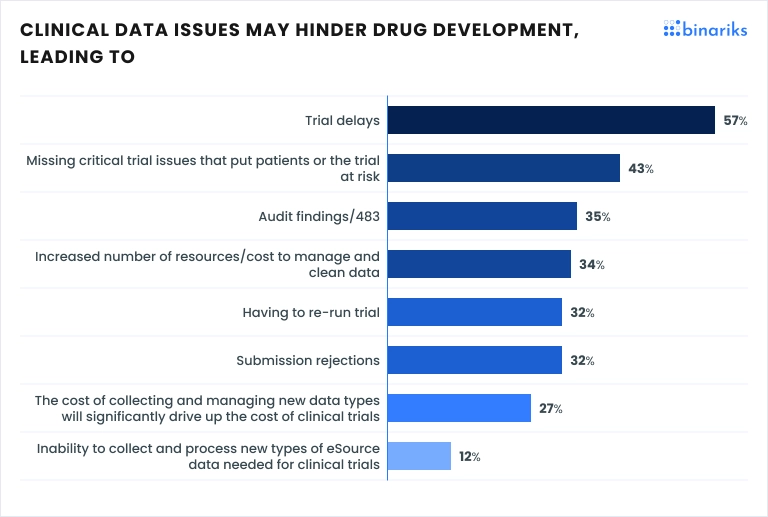
This article explores how modern data analytics can streamline processes, enhance accuracy, and improve cross-department collaboration. Drawing from real-world case studies, you'll learn:
- Why outdated practices lead to inefficiencies in trial execution and how to overcome them
- The benefits of integrating data analytics to boost clinical trial design and outcomes
- Practical tips for implementing advanced technology in your data analysis
Read to discover how data analytics can help you run more efficient and successful clinical trials.
The crucial role of data in the execution of clinical trials
Data is the backbone of clinical research, driving every stage from study design to final analysis. In the complex environment of clinical trials, accurate and comprehensive data collection is essential for making informed decisions that ensure patient safety and trial efficacy.
As the volume and complexity of data increase, clinical trial data analysis becomes even more critical in identifying patterns, predicting outcomes, and refining methodologies.
The transition to digital platforms has significantly enhanced the efficiency and accuracy of clinical research. By leveraging clinical data analytics, pharmaceutical companies can process vast amounts of data faster and more precisely.
This shift not only accelerates the development and approval of new drugs but also improves patient outcomes by ensuring that treatments are backed by robust, real-time data. In fact, industry research reveals that 97% of companies plan to expand the use of real-world patient data to further enhance the accuracy of their decision-making processes, reinforcing the inspiring role of data in driving successful clinical trials.
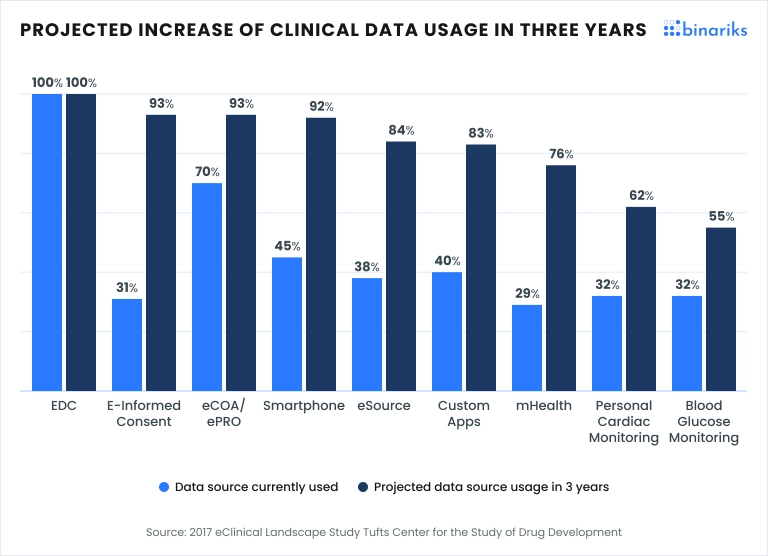
Additional considerations to keep in mind
- Improved data integrity: Digital platforms help maintain data integrity and traceability, reducing errors and ensuring compliance with regulatory standards.
- Enhanced decision-making: Advanced clinical trial analytics tools enable more informed and timely clinical trial decision-making, which can lead to faster adjustments and improved trial outcomes.
- Cost efficiency: Automated data collection and analysis reduce the need for manual intervention, cutting costs and minimizing the risk of human error.
- Real-time monitoring: Continuous data integration allows for real-time monitoring of trials, leading to quicker responses to adverse events and better overall management.
By focusing on these areas, companies can maximize the benefits of digital transformation in clinical trials, ultimately bringing life-saving drugs to market more efficiently.
Implementing technology for data analysis in clinical trials
The integration of technology into data analysis in clinical trials is transforming the landscape of clinical research. Advanced digital tools and platforms enable more precise and efficient data collection, management, and analysis, leading to better outcomes. Below is a detailed overview of key solutions and technologies being implemented in this direction:
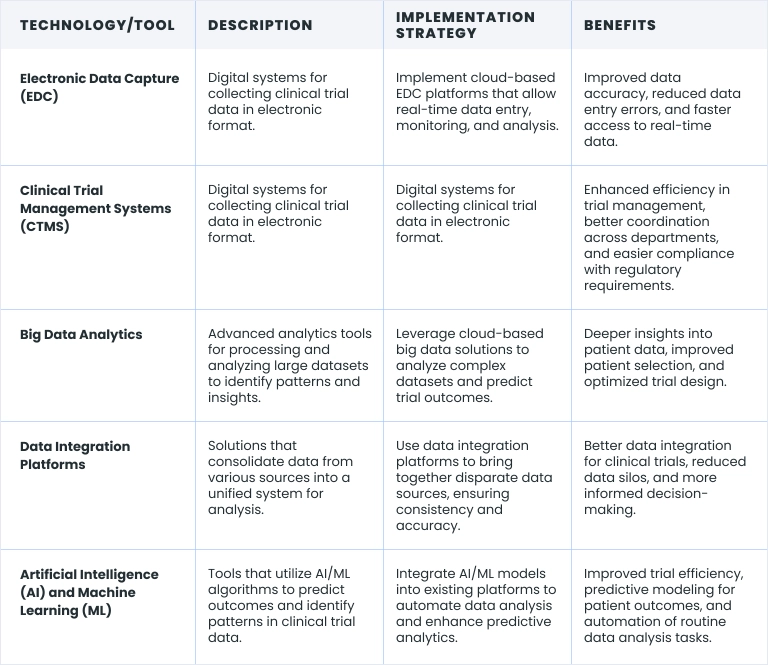
At Binariks, we specialize in developing and implementing these cutting-edge technologies to enhance data-driven decisions in clinical trials. Our expertise spans building solutions for big data analytics in clinical trials, integrating AI/ML models for predictive analytics , and designing comprehensive data integration platforms.
With a proven track record of helping different companies optimize their work, Binariks ensures that your trials are not only efficient but also grounded in accurate, real-time data.
By choosing Binariks as your partner, you gain access to our extensive experience and technical expertise. Together, we can overcome the challenges of modern clinical trials and achieve successful, data-driven results.
How can digital data sources enhance trial accuracy?
Digital data sources have significantly enhanced the accuracy and efficiency of clinical trials. By integrating data analytics in clinical trial design, pharmaceutical companies can leverage digital data to improve every aspect of trial execution. This section explores how these sources contribute to trial accuracy.
- Enhanced data collection and integration: Digital data sources like electronic health records (EHRs) and wearable devices provide a continuous flow of real-time data. This reduces manual data entry errors and improves data integration across platforms. Leveraging data analytics for the analysis of clinical trials ensures that this data is accurate and actionable.
- Improved patient selection and recruitment: Digital data allows for more precise patient selection by analyzing demographics and medical history. This leads to more effective recruitment and a better-matched trial population. Data-driven decision-making in clinical trials improves recruitment efficiency, reducing time and costs.
- Real-time monitoring and adjustments: Digital data enables real-time monitoring of patient progress, allowing for immediate adjustments to the trial protocol when needed. This capability enhances the overall accuracy and reliability of trial outcomes.
- Enhanced data accuracy and integrity: Automated systems and advanced analytic s maintain high data accuracy and integrity levels, ensuring compliance with regulatory standards and the validity of trial results.
- Optimized data analysis and reporting: Digital data sources facilitate faster and more comprehensive data analysis, supporting informed decision-making and leading to more successful trials.
Optimizing trial outcomes through advanced data management
Effective data management is essential for improving clinical trial quality, speed, and reliability. By adopting advanced strategies, clinical trial teams can streamline processes and achieve better outcomes. Here's how:
- Centralized data repositories: Implementing centralized repositories ensures secure, unified access to all trial data, facilitating seamless integration and reducing errors.
- Automation of routine processes: Automating tasks like data entry and validation minimizes human error and accelerates processes, ensuring precise data analytics in clinical trial design and analysis.
- Enhanced data security and compliance: Advanced systems offer robust security measures and ensure regulatory compliance, protecting sensitive patient information and maintaining data integrity.
- Real-time data access and collaboration: Providing real-time access to data enhances collaboration and supports decision-making, leading to more efficient outcomes.
- Scalability and flexibility: Scalable systems can adapt to the evolving needs of trials, enabling researchers to extract valuable insights and achieve successful results.
These strategies ensure that clinical trials are managed effectively, leading to more reliable and successful trial outcomes.
How do digital tools play a role in enhancing clinical trial efficiency?
Integrating digital tools into clinical trials has transformed the research process, making it more efficient and accurate. Advanced technologies allow pharmaceutical companies to streamline everything from patient recruitment to data analysis, which results in faster, more reliable outcomes and reduces the risk of errors that can occur with manual data entry.
Real-time data collection and monitoring capabilities allow researchers to make informed, data-driven decisions in clinical trials, which is essential for accelerating drug development and improving patient care.
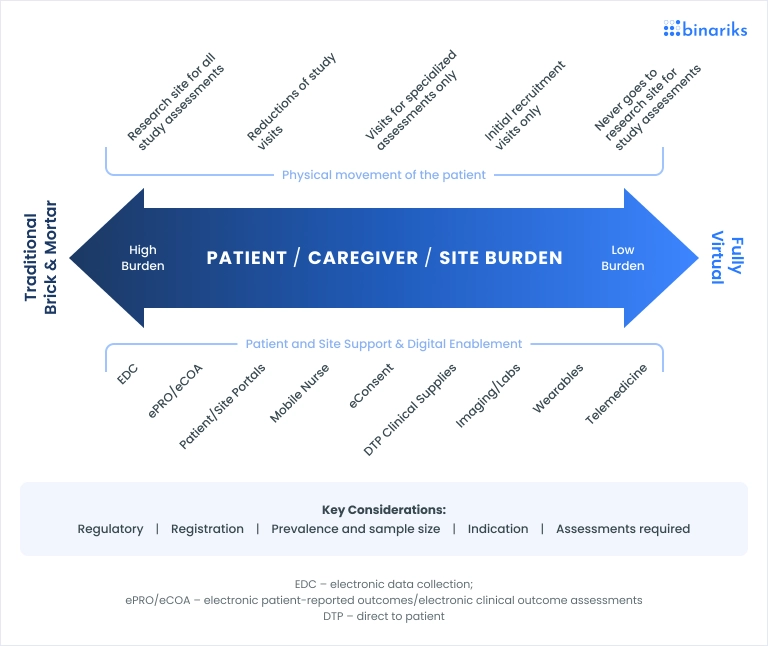
One of the most impactful advancements in this area is using digital therapeutics and pharma solutions. These tools enable continuous patient monitoring, providing real-time data that can be analyzed instantly. This allows trial protocols to be adjusted on the fly, ensuring that the study remains relevant and effective as new data emerges.
The ability to respond quickly to data trends not only improves the chances of a successful trial but also enhances patient safety by allowing for immediate interventions if adverse events occur.
Final thoughts
Integrating advanced data analytics and digital tools has become essential in optimizing clinical trials. These technologies enhance accuracy, streamline processes, and ultimately accelerate the development of new drugs, improving patient outcomes. Pharmaceutical companies can conduct more efficient and effective trials by adopting these innovations.
At Binariks, we are passionate about helping companies take full advantage of technological advancements. Our team works closely with you to implement tailored data analytics and digital tools that fit your unique needs, ensuring your clinical trials run smoothly and deliver results. Contact us, and let's work together to bring your innovations to life faster and more efficiently.
Share

