The drug development process is long and tedious, often taking about seven years for clinical trials and up to ten years for research and development. A significant part of this involves pharmaceutical document management, which requires handling large volumes of critical data across all stages of drug development.
One of the most significant operational burdens is document management in the pharmaceutical industry. Many documents for pharmaceutical document storage are still created and registered manually. Teams still largely rely on siloed systems and human data entry, where they must constantly reconcile data across various departments.
And it's not just pharma that has these issues. Industries operating under intense regulatory oversight, such as healthcare, insurance, and finance, grapple with similar documentation pain points. Their workflows are filled with repetitive manual tasks that are error-prone. The delays caused by these workflows lead to less revenue and a much slower innovation pace, which makes a business less competitive.
This article explores how automation, including intelligent document processing (IDP), can change the status quo, transforming and streamlining document management in the pharma industry. By removing inefficiencies and reducing human error, pharmaceutical document management helps pharmaceutical companies and other compliance-heavy industries improve the efficiency of their operations.
Want to dive deeper into document processing in general? Check out this article on Intelligent Document Processing (IDP), where our ML engineer answers FAQs, tackles challenges, and shares strategies for mastering IDP systems.
Handling a massive amount of documents
The primary reason manual pharmaceutical document management takes so long and is prone to issues is the myriad of documents in pharma: internal documents, external documents, and clinical study documents like CSR and clinical summary reports. Let's try to make sense of their meaning and determine at which stages to use them.
Internal documents
The company creates and maintains many internal documents to ensure compliance, operational efficiency, and quality assurance. As a rule, these docs are not shared with external parties.
- Standard Operating Procedures (SOPs): Detailed guidelines for processes like manufacturing, quality control, and document management.
- Batch records: Track production and testing of each batch to ensure traceability and compliance.
- Employee training records: Verify staff qualifications and training on processes, equipment, and compliance.
- Quality assurance documents: Include deviation reports, Corrective and Preventive Actions (CAPAs), and internal audits to comply with GMP standards.
- R&D documentation: Lab notebooks, research protocols, and formulation report are critical for early-stage development.
- Validation documents: Cover protocols and reports for process, equipment, and software validation, including IQ (Installation Qualification), OQ (Operational Qualification), and PQ (Performance Qualification).
- Change control records: Document modifications to processes, equipment, or procedures, ensuring controlled and approved implementation.
- Risk management files: Include risk assessments, Failure Mode and Effects Analysis (FMEAs), and risk control strategies.
- Inventory records: Track materials, components, and finished products for supply chain management.
- Environmental monitoring records: Document data on temperature, humidity, and other conditions in manufacturing and storage areas.
External documents
- Regulatory submissions: Applications like INDs (Investigational New Drug), NDAs (New Drug Applications), and CTAs (Clinical Trial Applications).
- Vendor agreements and contracts: Manage relationships with suppliers, manufacturers, and service providers.
- Patient information leaflets: Provide usage and safety instructions for patients as part of marketing authorizations.
- Regulatory inspection reports: Record findings from external audits and inspections by regulatory authorities like the FDA or EMA.
- Pharmacovigilance reports: Include Individual Case Safety Reports (ICSRs) and Periodic Safety Update Reports (PSURs).
- Advertising and promotional materials: Submitted for pre-approval to regulators to ensure compliance with promotional guidelines.
- Distributor and licensing agreements: Define terms for product distribution and licensing to third parties.
- Health authority correspondence: Official communications such as inquiries, deficiency letters, and responses.
- Certificates of Analysis (CoA): Verify that products meet quality and safety standards for regulatory compliance and distribution.
- Publication materials: Articles, abstracts, and posters are shared in journals or conferences to disseminate findings.
Clinical study documents
Essential for conducting and reporting clinical trials, these documents ensure regulatory compliance and data integrity.
- Clinical Study Report (CSR): Detailed analysis of clinical trial data, adhering to ICH E3 guidelines.
- Clinical summary report: High-level summary of the CSR, focusing on key safety and efficacy findings.
- Investigator brochures (IBs): Summarize preclinical and clinical data on investigational drugs for investigators.
- Clinical Data Management Plans (CDMPs): Outline methods for data collection, cleaning, and analysis during clinical trials.
- Informed Consent Forms (ICFs): Document patient consent to participate in trials, including risks and benefits.
- Site monitoring reports: Records of site visits and evaluations to ensure compliance with trial protocols.
- Statistical Analysis Plans (SAPs): Define methodologies for analyzing clinical trial data.
- Protocol amendments: Document approved changes to trial protocols during the study.
- Periodic safety reports: Provide updates on patient safety and adverse events to regulatory authorities.
- Trial Master File (TMF): Comprehensive collection of documents required for clinical trial conduct and regulatory compliance.
Documents at key drug development stages
These documents evolve across the lifecycle of drug development.
Discovery and preclinical
At this stage, the focus is on preparing an Investigational New Drug (IND) application to the Food and Drug Administration (FDA). The documents used are:
- Research protocols and data records: Document experimental designs and results.
- Toxicology reports: Summarize preclinical safety studies.
Clinical trials
During the clinical trial, New Drug Application (NDA) submissions are prepared. Some new documents that appear at this stage are:
- Trial protocols and site qualification reports: Define study objectives and ensure site compliance.
- Patient consent forms and investigator agreements: Ensure ethical and legal trial conduct.
Manufacturing
- Batch records and cleaning validation reports: Ensure product quality and cross-contamination control.
- Equipment maintenance logs: Track routine servicing to maintain operational standards.
Marketing authorization
- Regulatory submission dossiers: Comprehensive documentation for product approval.
- Summary of Product Characteristics (SmPC): Provide healthcare professionals with essential drug information.
Post-market surveillance
- Adverse event reports: Monitor and document drug safety after market approval.
- Periodic Benefit-Risk Evaluation Reports (PBRERs): Assess long-term product safety and efficacy.
Benefits of document management automation in pharma
Automated pharma document management solves many issues with documents, mainly the tedious and expensive generation of clinical study reports that prevent scientists from focusing more on the scientific aspects of clinical trials. Here are the main benefits detailed:
Time savings
Efficient document management automation dramatically reduces the time spent on document-related processes and improves time to market.
- Instant retrieval of adverse event reports or historical clinical trial data ensures rapid responses to regulatory queries.
- Tasks like approval processes, regulatory submissions, and report generation are streamlined with automated notifications and routing.
- Automation minimizes repetitive administrative tasks like manual data entry and formatting so scientists can focus on higher-value activities.
- Automated preparation and submission of documents in formats like eCTD make regulatory hurdles faster. Some time-consuming tasks, like manually documenting the entire clinical trial, take time away from potentially life-saving drug development. Automatic documentation speeds up FDA approval.
- AI in pharma R&D and big data analytics in pharma can speed up document retrieval and improve data integration across trials.
Improved accuracy
Automation reduces human error, which is critical in maintaining compliance and ensuring patient safety.
- Automated systems validate inputs and reduce manual errors during data entry and document updates. Textual redundancy is decreased as well.
- Version control ensures that only the latest, approved versions of documents are available.
- Automated validation checks align documents with industry standards such as FDA, EMA, or ICH guidelines.
- Comprehensive audit trails for all actions (creation, modification, and access) make documents error-proof and traceable for inspections.
- The quality is better thanks to advanced templates.
- Document automation in pharma allows us to find and quote earlier research easily.
Convenient access
Automation enhances accessibility, ensuring people can access the right documents at the right time.
- A centralized repository means all documents are stored in a single, cloud-based, or on-premise system, accessible across departments. This enables secure document storage for pharmaceutical companies.
- Role-based access control ensures sensitive information is available only to authorized personnel.
Enhanced collaboration
Document management automation facilitates better teamwork within and across departments. Moreover, clinical trials are often carried out across multiple locations worldwide, and document automation in pharma helps ensure that no entry is missing.
- Teams can collaborate on documents in real time, reducing delays caused by manual edits and version conflicts. Any language barrier between scientists in different countries is effectively eliminated.
- Documents can be securely shared with external partners.
- Templates and consistent formatting promote uniformity across documents.
Cost efficiency
Automation leads to significant cost reductions across multiple areas.
- Accurate documentation prevents costly regulatory violations.
- Reducing delays in clinical trial documentation and regulatory reviews prevents revenue losses due to missed drug approval deadlines.
A purpose-built document management software for the life sciences industry not only improves compliance but also delivers long-term cost savings by reducing manual workload and approval cycle time.
Challenges of document automation
Pharmaceutical document management challenges are inevitable, whether you decide to streamline or not. Here are the common issues encountered by developers at Binariks and the best ways to solve them:
Challenge 1: Tracking and comparing multiple versions of drug documentation
Pharmaceutical documentation for a single drug may be submitted multiple times with updates. For example, a drug released in 2022 might have an update about the manufacturer in 2023 and changes regarding side effects in 2024. Tracing and comparing these versions is essential to ensure accuracy and compliance.
Solution:
- Document branching: A version tree for drug documentation allows tracking of when each version was created and viewing its details. Users can also add comments to specific versions for better context.
- Comparison module: A side-by-side comparison tool highlights changes between two document versions.
- AI-generated summaries: Automatically generate summaries of changes between document versions for quick understanding.
Industry parallel: In clinical trials, changes in protocol amendments must also be tracked and justified across submissions, just like SmPC updates.
Challenge 2: Collaborative work on pharma documentation
Pharmaceutical documents often involve multiple contributors. For instance, someone drafts the document, others review and approve it, and translators may also be involved in multi-market releases.
Solution:
- Workflow management: Implement workflows with key steps, from drafting to final documentation.
- Task assignment and notifications: Assign tasks to users, with notification systems for tracking to-do items, overdue items, and approvals. Support for electronic signatures and 2FA enhances security.
- User roles and permissions: Define roles with specific permissions, ensuring users work only on assigned drugs rather than having access to all company projects.
Industry parallel: Medical billing departments must restrict access to Protected Health Information (PHI) based on HIPAA roles, route claims through claim clearance, and maintain traceable documentation trails for insurance audits or CMS reviews.
Challenge 3: Error validation before regulatory submission
Errors in documentation can lead to rejections or multiple submission attempts to regulatory bodies. Automation supports pharmaceutical document verification, helping documents meet FDA and EMA guidelines before submission.
Solution:
- Custom validation rules: Implement rules based on regulatory guidelines (e.g., FDA’s implementation guide) to validate documents before submission. Provide user-friendly hints to guide corrections.
- Integration with error analysis tools: Identify and resolve potential errors using existing tools.
Industry parallel: In finance, regulatory filings (e.g., Sarbanes-Oxley disclosures) must comply with strict formats. Errors can result in SEC fines or loss of investor trust.
Challenge 4: Managing complex and extensive drug documentation
Drug documentation is vast, making it easy for authors to overlook details, make errors, or mislink sections. AI-based tools streamline pharmaceutical document processing, making SmPC and PIL files consistent.
Solution:
- AI-powered coherency and consistency checks: Use NLP tools to ensure:
- Correct spelling and grammar.
- Accurate naming and dosage of the drug across the document.
- Logical connections between statements.
- The presence of referenced illustrations or tables.
- Valid links to correct sections (e.g., a link to Section 12 points to the correct section).
Industry parallel: In finance, prospectuses and investment memoranda contain interlinked sections (risk, performance, compliance, disclosures) where a single inconsistency may cause regulatory pushback.
Challenge 5: The patient information leaflet (PIL) bottleneck
One of the clearest examples of pharma's documentation burden is the Patient Information Leaflet (PIL), a legally required, EU-compliant document derived from the Summary of Product Characteristics (SmPC). The manual transformation of SmPCs into PILs really benefits from regulatory document automation for pharma.
To illustrate this challenge, let's look at a real-world example.
Binariks worked with a client, a mid-sized European pharmaceutical company that used to spend 2-3 weeks per product creating PILs manually. This caused cascading delays in market launches and left room for human error even in "routine" leaflets.
Binariks' solution:
- AI-powered PIL generation, based on SmPC parsing and Copilot-driven content creation.
- Rule-based logic for EU formatting and readability requirements (Directive 2001/83/EC).
- Human-in-the-loop validation
- Automated compliance checks
Industry parallel:
- Clinical trials: Writing and localizing investigator brochures or informed consent forms.
- Insurance: Generating compliant claim summaries from policy data.
- Finance: Assembling market disclosures from structured data under strict timelines.
Challenge 6: Scaling documentation across products and markets
Manual processes fall apart when a company must launch multiple drugs in the same family or in various regions. It happens not just because of the sheer scale but also because of factors like regulatory fragmentation with many different laws in place and linguistic barriers.
Organizations expanding across markets need cloud-based solutions for pharma document management to manage multilingual compliance and version control.
Solution:
- Process standardization: Use AI-enhanced templates to enforce structure, terminology, and formatting across all products.
- Scalable architecture: Enable batch processing of related documents, with smart version control and multilingual capabilities.
- Integrated feedback loops: Refine the system using real-world edits and reviewer comments to improve over time.
Industry parallel: Global banks and insurers must also generate multi-market documents with localized regulatory language, which is a natural use case for automation + human review.
Choosing and implementing document management systems in pharma
Implementing a Document Management System (DMS) can revolutionize how documents are handled, but selecting and deploying the right system requires careful evaluation. Here's a breakdown of key considerations:
Evaluating the need for a document management system
Assessing the company's specific requirements and challenges is essential before adopting a document management system in the pharmaceutical industry.
- Does the system meet industry standards such as FDA 21 CFR Part 11, GDPR, and ICH guidelines?
- Can the system handle growing document volumes as the company expands?
- Does the organization face bottlenecks in approvals or version control?
- Is there a lack of standardized document control in the pharmaceutical industry that leads to delays?
- Is the system compatible with existing software such as ERP, LIMS, or quality management tools?
Criteria for selecting a DMS
When choosing a DMS for pharmaceutical operations, consider the following factors:
- Ensure the system supports validation and includes features like electronic signatures and audit trails.
- Make sure the system is easy to use and has intuitive interfaces and minimal learning curves.
- Choose a system that can be tailored to fit your company’s unique workflows and document types through customization.
Steps for implementing a DMS in pharma
- Engage teams from QA, regulatory affairs, IT, and operations to align goals and identify key requirements.
- Develop a strategy to transfer existing documents without compromising data integrity or losing important information.
- Validate the system to ensure it meets compliance standards and works as intended.
- Provide comprehensive training for all users.
- Conduct pilot testing before full deployment and monitor performance to identify and resolve issues early.
- After employing the system, keep it up to date to meet evolving regulatory requirements and industry best practices.
- Use the DMS to maintain accurate audit trails for inspections and regulatory reviews.
Popular document management systems for pharma
Several DMS options cater specifically to the pharmaceutical industry's needs. AI-driven tools, like AI in pharmacovigilance , enable advanced error detection and workflow optimization.
- MasterControl Document Management: Offers robust validation and collaboration tools tailored for life sciences and pharma.
- OpenText Documentum: A scalable solution with advanced security features and integration capabilities for regulated industries.
- TrackWise Digital: Combines document management with quality management.
- DocuWare: A user-friendly DMS suitable for mid-sized pharma companies, with features like secure access and mobile compatibility.
Drive pharma innovation with Binariks' tech expertise!
AI in pharma: The compliance-first approach
AI can dramatically speed up and simplify pharmaceutical document management, but not all AI is built for regulated environments. In heavily regulated industries like pharma, compliance must come first, not the AI model, no matter how convenient it seems.
For business executives dipping their toes into AI for pharma, it is tempting to think that a general-purpose language model like ChatGPT can solve documentation bottlenecks on its own.
The truth is that ChatGPT and similar general-purpose LLMs are not compliant out of the box for regulated industries like pharma because there is no built-in traceability. They are also not built within the regulatory rule framework of the pharma industry.
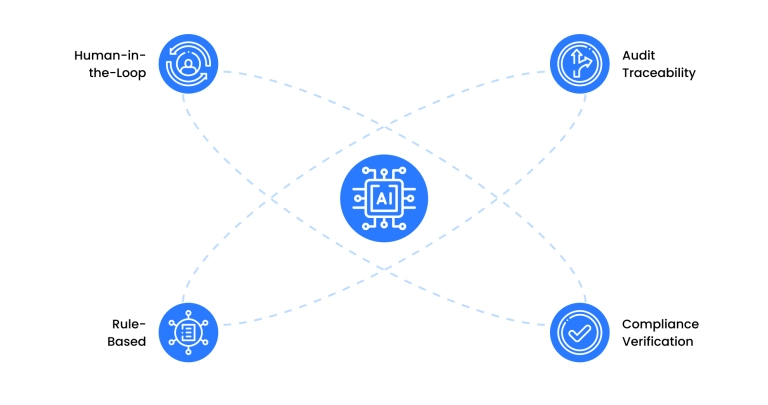
What makes an AI model regulatory compliant is a system architecture designed around regulatory constraints. This includes:
- Human-in-the-loop (HITL) validation
AI can draft and structure content, but human subject matter experts remain essential for verifying sensitive content in PILs, SmPCs, CTDs, and labeling documents for regulatory alignment and prevention of AI hallucinations.
- Rule-based frameworks aligned with regulation
Compliant AI systems are grounded in explicit regulatory logic, instead of just predictions. For example, transforming SmPCs into PILs requires EU Directive 2001/83/EC adherence.
- Automated compliance verification
AI-powered systems can scan outputs for:
- Missing sections
- Terminology inconsistencies
- Formatting violations
This is done to catch issues early before human review or regulatory submission.
- Audit readiness and version traceability
To meet GxP and 21 CFR Part 11 requirements, AI outputs must be:
- Traceable (when and how the AI was used)
- Explainable (which inputs led to which outputs)
- Version-controlled (what changed, who validated it, when)
How we tackle document management challenges
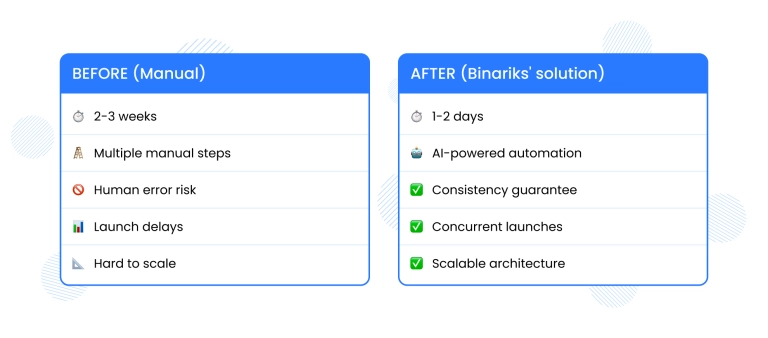
A mid-sized European pharmaceutical company approached Binariks with a bottleneck. Their manual creation of EU-compliant Patient Information Leaflets (PILs) was taking 2-3 weeks per product.
With multiple product launches happening simultaneously, the delays affected the company's time-to-market and overall operational efficiency.
The client relied on regulatory teams to manually convert Summary of Product Characteristics (SmPCs) into patient-friendly PILs. The process was time-consuming, prone to error, and difficult to scale while consuming many resources.
Solution
We determined that the PILs could be efficiently generated directly from the Summary of Product Characteristics (SmPC) documents.
As a solution, Binariks developed a custom pharma document management system powered by AI automation with a compliance-first architecture. AI-powered PIL generation project combined Microsoft Copilot, rule-based logic, and Human-in-the-Loop validation.
Here are the key elements of the solution:
- Document processing engine for parsing SmPCs in various formats
- Rule-based transformation logic aligned with EU Directive 2001/83/EC
- AI-enhanced content generation using Microsoft Copilot as the AI foundation with rule-based logic specifically designed for EU pharmaceutical regulations.
- Built-in human validation cycles involving regulatory experts
- Automated compliance verification
- Scalable architecture ready for future expansion to new document types and regional requirements
This wasn't a one-size-fits-all implementation, but a collaborative process involving domain experts, regulatory teams, and AI/ML engineers. The project was broken into several phases. For development, we used Agile methodology with continuous feedback integration.
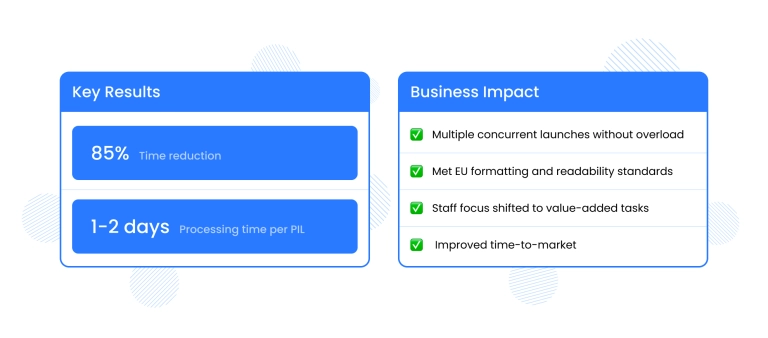
The results
- 85% reduction in processing time: PIL creation time dropped from 2-3 weeks to 1-2 days
- Improved accuracy with consistency and removed errors
- Regulatory compliance achieved, with all PILs passed internal QA and met EU formatting and readability standards
- The client is now able to manage multiple concurrent launches without overloading their teams
- Regulatory staff can now focus on value-added tasks instead of document formatting
Conclusion
The drug development process relies heavily on managing vast documents across all stages, including internal, external, and clinical study records. Automation offers significant advantages, streamlining workflows, improving accuracy, and saving valuable time.
While it may not address all challenges, such as complex regulatory changes or nuanced tasks requiring human judgment (like resolving data discrepancies, ensuring contextual accuracy and ethical appropriateness, etc.), automation is a powerful tool when strategically implemented alongside manual expertise.
At Binariks, we understand the complexities of pharmaceutical document management and when manual expertise is required to complement automated systems.
That's why we take a compliance-first approach, combining AI-powered tools with rule-based logic and Human-in-the-Loop validation – just as we did when helping a European pharma company reduce Patient Information Leaflet (PIL) creation time by 85%.
Our experts create tailored solutions that integrate seamlessly with existing tools and workflows. And it's not just in pharma. Healthcare, insurance, and finance face similar documentation pain points.
By prioritizing scalability, adaptability, and compliance, your organization can harness automation to meet evolving regulatory demands, enhance operational efficiency, and support sustainable growth. With the right approach, automation becomes a key driver for innovation and success in the pharmaceutical industry.
FAQ
Share

