Efficient data management is a critical component of successful clinical trials. Electronic Data Capture (EDC) systems have become a standard tool, replacing outdated paper-based methods to improve data collection accuracy, compliance, and speed. By implementing EDC in clinical trials, researchers can collect and manage data electronically, making the process more streamlined and reducing the risk of errors.
These systems offer a secure and reliable solution for handling large volumes of data, ensuring that clinical trials run smoothly while meeting regulatory standards. Clinical trial EDC platforms also enable faster access to data for analysis and reporting, accelerating the overall timeline for research and helping bring new treatments to patients sooner.
By reading this article, you will understand:
- The function and purpose of EDC in clinical trials;
- Key advantages of using EDC systems in research;
- Common challenges and limitations of these systems;
- How EDC improves data accuracy and compliance;
- Examples of widely used EDC software for clinical trials;
- Best practices for implementing an EDC system;
- How to choose the right EDC solution for your trial.
Read on to explore how clinical trial EDC systems can enhance your research processes and ensure the success of your clinical studies.
What is Electronic Data Capture (EDC) in clinical trials?
Electronic Data Capture (EDC) systems are digital platforms designed to collect and manage EDC clinical data during clinical trials. They replace manual, paper-based methods, providing a more efficient and accurate way to handle vast research-generated data.
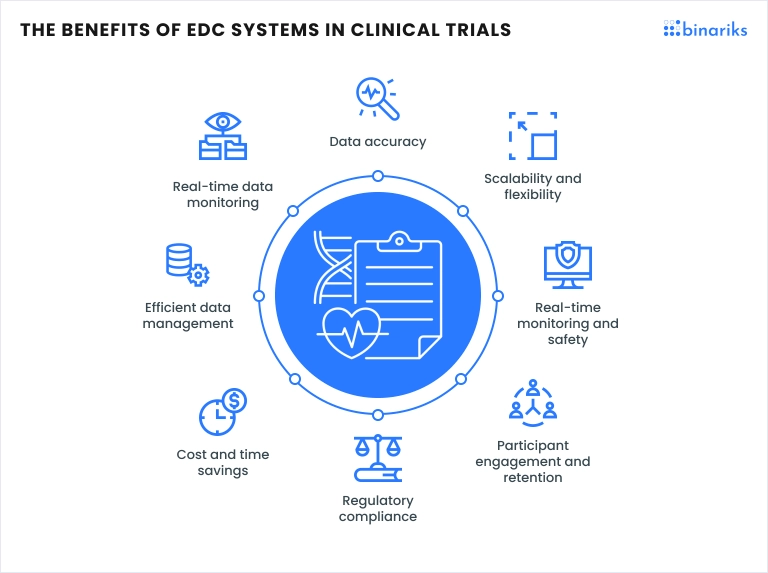
The role of EDC in clinical trial operations is critical to ensuring compliance with regulatory standards and improving data integrity. By automating data collection, EDC systems reduce the likelihood of human error and ensure that all relevant information is stored securely and can be easily retrieved at any point in the study. This ultimately enhances the reliability of trial outcomes and speeds up the process of bringing new therapies to market.
These systems are an integral part of modern clinical research, as shown in numerous EDC clinical trial examples that demonstrate their impact on improving data management and the overall efficiency of clinical trials.
Challenges and limitations of EDC systems
While EDC systems in clinical trials offer significant advantages, their implementation is not without challenges. The integration of these systems requires careful planning, investment, and training to ensure they function optimally within the broader clinical trial infrastructure. Below are some key challenges and limitations that researchers may face when adopting an EDC system for clinical trials.
The challenges include:
- The complexity of integration: Incorporating an EDC system for clinical trials into existing workflows can be complex, especially when integrating with other systems, such as clinical trial management systems (CTMS) or laboratory information systems (LIS). Ensuring compatibility across platforms and data sources can be time-consuming and requires technical expertise.
- High initial costs: Implementing EDC systems often involves significant upfront costs, including software licensing, customization, and IT infrastructure. Smaller organizations or studies with limited budgets may find this financial barrier difficult to overcome, especially if the full benefits aren't immediately realized.
- Personnel training requirements: The shift from paper-based methods to EDC software in clinical studies necessitates comprehensive training for the clinical staff. Learning how to effectively use EDC platforms requires time and resources, and there may be a learning curve before the system is fully adopted.
- Data migration and system validation: For organizations transitioning from legacy systems, migrating existing data into the new EDC platform can be challenging. This process must be carefully managed to ensure data integrity, and the system itself must be validated to comply with regulatory standards.
- Technical support and maintenance: EDC systems require ongoing technical support and regular updates to address software bugs and security vulnerabilities. Lack of timely support or updates can lead to operational disruptions and even security risks, especially when handling sensitive clinical data.
Addressing these challenges is critical for ensuring that EDC systems perform as expected and deliver the intended benefits during clinical studies.
How EDC systems improve data accuracy and compliance
The best EDC systems for clinical trials can enhance data accuracy by automating data entry and validation processes. These systems reduce human errors typically associated with manual data collection and ensure that all required fields are properly completed before data can be submitted. Automated checks and validation rules built into EDC platforms help researchers maintain clean, error-free data, ensuring the information collected is accurate and reliable.
Compliance with regulatory standards is another significant advantage of EDC in clinical data management. EDC systems automatically generate audit trails, tracking every modification made to the data, which simplifies maintaining compliance with guidelines like GCP and FDA regulations. These systems also support secure storage and encryption, ensuring patient information is protected, and clinical trials meet strict data privacy standards.
Features of a clinical EDC system
EDC systems for clinical research are designed with multiple features that enhance data management during clinical trials. These tools enable researchers to collect, store, and analyze data while maintaining regulatory compliance. Below are some of the key features found in these systems:
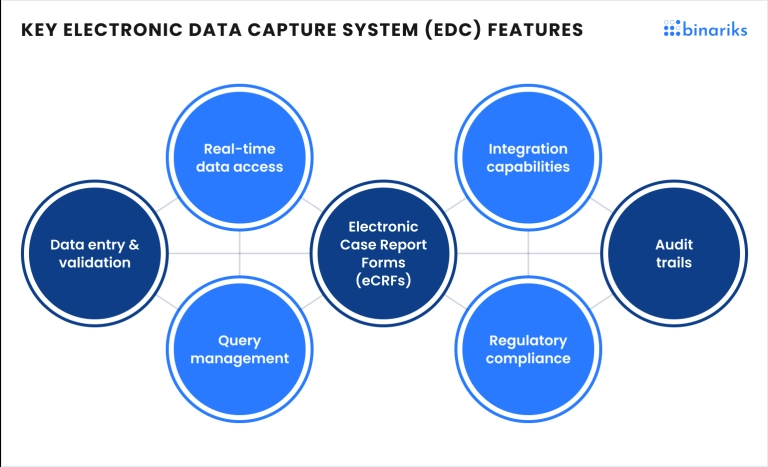
- Real-time data entry: Researchers can input data as soon as it is collected, enabling timely access to information and faster trial decisions.
- Data validation and error-checking: Automated validation rules ensure that the data entered meets specific criteria, reducing the occurrence of manual errors and improving overall data quality.
- Audit trails: Electronic data capture systems (EDC) in clinical trials maintain detailed audit trails that log every change made to the data. This feature is crucial for maintaining transparency and meeting regulatory standards.
- Secure access and storage: EDC systems provide encryption and role-based access controls to safeguard sensitive clinical data, ensuring that only authorized personnel can access or modify it.
- Reporting and analytics tools: These tools allow researchers to generate customized reports and perform real-time data analysis, which can greatly improve trial efficiency and data-driven decision-making.
- Multi-site support: EDC systems often support data collection across multiple sites, ensuring that trials conducted in different locations can seamlessly feed data into a central platform, facilitating coordination and data consistency.
- Remote monitoring capabilities: Many EDC systems allow for remote data monitoring, giving sponsors and CROs the ability to oversee trials without being on-site, helping reduce operational costs, and improving oversight.
- Flexible study design options: EDC platforms typically offer flexible study design features, allowing researchers to easily adjust study protocols and adapt to new requirements as trials progress, making the system more versatile for different phases of research.
These features collectively support the efficient conduct of clinical trials, helping researchers manage data more effectively.
Drive pharma innovation with Binariks' tech expertise!
Examples of EDC software for clinical trials
Here are three widely used EDC software solutions for clinical trials, each offering unique advantages and some limitations:
Castor EDC
Castor EDC is a cloud-based EDC platform designed to simplify data capture and management for both small and large-scale clinical trials.
- Pros: User-friendly interface with drag-and-drop functionality; supports rapid setup and deployment; affordable for smaller studies.
- Cons: Limited customization options compared to more complex systems; advanced features can be expensive for larger trials.
ClinCapture
ClinCapture offers a flexible, cloud-based EDC system that allows sponsors to build their studies with minimal programming effort, making it ideal for quick launches.
- Pros: Free for basic use with a tiered pricing model; easy to use and configure without extensive technical knowledge.
- Cons: Some features, like advanced reporting, require higher-tier subscriptions; may not be suitable for highly complex trials.
Viedoc
Viedoc is known for its modern, intuitive design and strong compliance with international regulatory standards, making it a favorite for global clinical trials.
- Pros: Excellent user experience with an intuitive design; strong support for global trials, including multilingual capabilities.
- Cons: Higher cost for advanced features; may require more customization for niche studies.
These EDC platforms play a crucial role in ensuring data integrity and compliance. Their integration is especially important when considering the broader role of data analysis in clinical research , where accurate data management is a key to trial success.
Best practices for EDC implementation for clinical trials
At Binariks, we know that the success of an EDC implementation relies on careful planning and the right approach. Here are some simple tips for ensuring a smooth and efficient EDC rollout for your clinical trial:
- Plan for seamless integration
Ensure your EDC system integrates with other essential platforms, such as Clinical Trial Management Systems (CTMS) and Laboratory Information Systems (LIS). Without proper integration, data silos can form, leading to inefficiencies and errors. A well-integrated system allows for smooth data flow and enhances overall trial management.
- Invest in comprehensive user training
The success of any EDC system heavily depends on the users. Training your entire team, from data entry staff to clinical investigators, is essential to use the system effectively. Proper training reduces the likelihood of mistakes, increases productivity, and ensures everyone is confident with the new technology.
- Prioritize data security
Security is a critical aspect of EDC implementation. Look for a system that provides robust encryption, secure logins, and role-based access controls. Ensuring that clinical data is protected maintains compliance with regulations and safeguards patient information from potential breaches.
- Ensure regulatory compliance
Regulatory compliance is non-negotiable in clinical trials. Your EDC system must meet international standards, such as FDA or EMA guidelines, and include features like audit trails and data validation. Compliance ensures your trial data is trustworthy and that your research can proceed without regulatory obstacles.
- Validate the system thoroughly
Before fully implementing the EDC platform, performing system validation is vital. This involves testing to ensure the system meets all functional requirements and regulatory standards. Proper validation ensures that the system runs smoothly and can handle the complexities of your clinical trial.
- Take time for proper implementation
While there may be pressure to move quickly, rushing through EDC implementation can lead to costly mistakes. Taking a thoughtful, step-by-step approach—covering configuration, data migration, and testing—will yield better results and minimize the risk of errors later in the trial.
By following these practices, you can ensure that your EDC system not only supports your trial's objectives but also enhances the overall efficiency and reliability of your clinical data management.
How to choose the right EDC system for your clinical trial
Selecting the right EDC system depends on the specific needs of your clinical trial. Consider factors like trial size, complexity, and regulatory requirements. Smaller trials may prioritize ease of use and cost, while larger, more complex studies might need advanced features like real-time data monitoring, multi-site support, and comprehensive security measures. Always make sure the platform complies with relevant regulations and integrates seamlessly with your existing systems.
At Binariks, we specialize in helping organizations choose and implement the best EDC solutions tailored to their unique clinical research needs. Our team can guide you through the selection process and facilitate smooth integration. Contact us today to learn how we can optimize your clinical trial’s data management with the right EDC system.
Share

