AI in pharmacovigilance is changing the way pharmaceutical companies monitor drug safety. Traditionally, pharmacovigilance relies on manual processes to collect, assess, and prevent adverse drug reactions. While effective, these methods are time-consuming and often struggle to keep up with the massive volumes of data generated.
In this article, we delve into how AI transforms traditional pharmacovigilance approaches, improving workflows and enhancing the detection of side effects. Readers will learn:
- The fundamentals of AI-enhanced pharmacovigilance;
- The benefits and tools available for safer drug monitoring;
- Real-world applications and challenges of implementing AI.
Read on to explore how AI is reshaping drug safety monitoring.
Brief introduction to pharmacovigilance
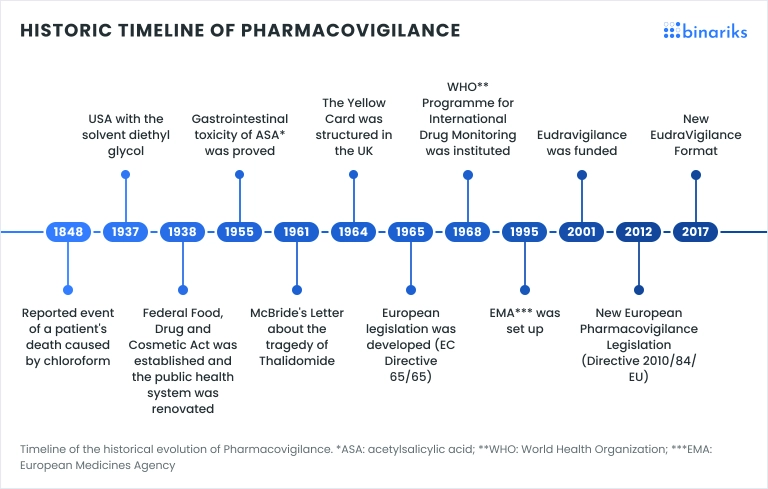
Pharmacovigilance ensures patient safety by monitoring, identifying, and preventing adverse drug reactions. Originating in the 1960s after incidents like the thalidomide crisis, it has evolved from manual reporting to more advanced methods.
The field began with manual processes focused on collecting and assessing drug safety data, which, while effective, had limitations in scope and efficiency. As technology advanced, AI transformed traditional pharmacovigilance approaches.
Where once data was collected manually, AI and pharmacovigilance now work together to automate complex data analysis, rapidly identify adverse events, and use predictive modeling for risk management.
These AI-driven changes mirror broader innovations in pharmaceuticals, as seen in AI in pharma R&D , where research, development, and drug safety are being conducted. By integrating pharmacovigilance with AI, real-time insights are now possible, improving the safety monitoring of drug therapies across the board.
What is AI-enhanced pharmacovigilance?
AI-driven pharmacovigilance refers to the use of artificial intelligence technologies to automate the detection, analysis, and management of adverse drug reactions, enhancing drug safety processes. It has significantly reshaped drug safety by streamlining case processing and monitoring.
According to a study published in ScienceDirect, using AI in pharmacovigilance has reduced case processing times by up to 50%, allowing quicker identification and analysis of adverse drug reactions and enhancing patient safety (Source ).
AI-driven automation also enhances regulatory compliance. Pharma Focus America notes that automated data analysis ensures timely and accurate safety reporting, while predictive models help proactively manage safety risks before they impact patients (Source ). This reflects the broader importance of AI in healthcare, where technology is revolutionizing patient outcomes and regulatory compliance across various medical fields. You can learn more about these advancements here .
Furthermore, AI-powered pharmacovigilance contributes to operational efficiency. A report by the National Center for Biotechnology Information (NCBI) highlights a 60% reduction in manual processing times for safety reports, reducing costs and improving resource use.
So, here are the key benefits of AI in pharmacovigilance:
- Faster detection: Quicker analysis of adverse events.
- Proactive risk management: Predictive analytics for early safety measures.
- Operational efficiency: Streamlined workflows and reduced costs.
Benefits of AI in pharmacovigilance
Leveraging AI in pharmacovigilance offers numerous advantages, transforming traditional drug safety workflows into more efficient and proactive processes. Here are the top five benefits.
1. Improved data analysis and signal detection
Pharmacovigilance AI solutions enable faster and more accurate analysis of large datasets from various sources, such as medical literature and social media. This leads to better signal detection for adverse drug reactions and potential safety issues, ensuring quicker risk responses.
2. Enhanced efficiency through automation
Implementing pharmacovigilance automation with AI significantly reduces manual data processing. This frees up time for pharmacovigilance professionals to focus on more strategic decision-making, streamlining workflows and boosting overall productivity.
3. Real-time monitoring and proactive risk management
AI-powered pharmacovigilance solutions provide real-time monitoring capabilities, allowing for the early detection of potential adverse effects. Predictive analytics help identify patterns, enabling proactive risk management before safety issues escalate.
4. Better compliance with regulatory requirements
Automated systems powered by AI ensure that safety reports are timely, complete, and in compliance with global regulatory standards. This reduces the risk of errors in documentation and allows for more consistent reporting, ultimately improving drug safety and company compliance.
5. Cost savings and resource optimization
The use of AI in pharmacovigilance reduces manual workload and operational costs. By enhancing data processing efficiency, companies can reallocate resources more effectively, improving productivity while maintaining safety and regulatory adherence.
Drive pharma innovation with Binariks' tech expertise!
AI tools and techniques in pharmacovigilance
Artificial intelligence in pharmacovigilance includes technologies and methods that enhance drug safety monitoring and decision-making. Key techniques such as machine learning, natural language processing, and robotic process automation play a vital role in improving data analysis and case management.
- Machine Learning Algorithms: ML uses historical data to train models that recognize patterns and predict potential adverse reactions. It adapts and learns over time, enabling accurate signal detection and risk assessment, making it central to artificial intelligence for pharmacovigilance.
- Natural Language Processing: A significant amount of data in pharmacovigilance is unstructured, like clinical notes, social media, and literature. NLP helps extract and organize this information, allowing AI to operate efficiently for adverse drug reaction monitoring.
Robotic Process Automation: By automating these processes, RPA streamlines repetitive tasks like data entry, case intake, and regulatory reporting. Combined with AI, it significantly reduces manual work and enhances data accuracy, freeing time for strategic pharmacovigilance activities.
Several platforms leverage these techniques:
- VigiBase: The World Health Organization's (WHO) global database of adverse drug reactions. It uses AI algorithms to analyze large volumes of safety reports and detect emerging patterns of adverse effects worldwide.
- EudraVigilance: The European Union's adverse reaction reporting system. It employs machine learning algorithms to identify potential safety signals from data submitted by patients and healthcare professionals, effectively addressing privacy concerns.
- SIGNAL: Developed by the IMI WEB-RADR Consortium, this tool uses AI models to predict potential risks associated with specific drugs, allowing for proactive safety interventions.
These AI-powered solutions for pharmacovigilance integrate advanced techniques, providing faster and more accurate safety surveillance, better compliance, and optimized resource use in drug safety workflows.
The workflow of AI-enhanced pharmacovigilance
AI is transforming pharmacovigilance processes by streamlining and automating key steps in the workflow. Here’s an overview of a typical AI-enhanced pharmacovigilance workflow:
- Data collection and integration: AI tools gather data from diverse sources, including medical records, social media, and patient reports, and then integrate it for comprehensive analysis.
- Signal detection and prioritization: Machine learning algorithms analyze the data for potential safety signals and prioritize them based on severity and frequency, enhancing the efficiency of risk identification.
- Risk assessment and regulatory reporting: AI systems assess the detected signals, evaluate associated risks, and generate reports for regulatory compliance, thereby automating pharmacovigilance reporting with AI for faster response times.
- Case management and follow-up: Automated case processing ensures that adverse events are tracked, followed up, and managed throughout their lifecycle.
- Continuous monitoring and feedback: AI-driven tools provide ongoing surveillance and feedback loops, constantly improving the accuracy of signal detection and risk management.
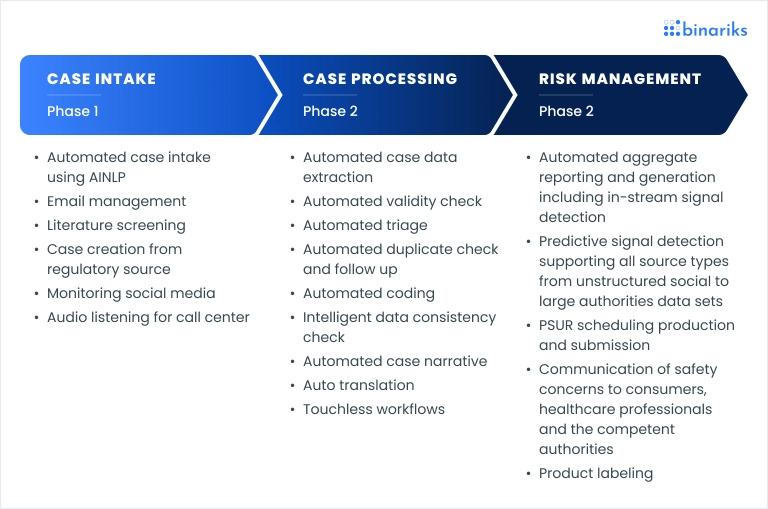
This workflow enables a faster, more efficient approach to pharmacovigilance, enhancing drug safety and regulatory compliance.
Challenges of implementing AI in pharmacovigilance
Implementing AI in pharmacovigilance for drug safety faces some unique challenges that go beyond general AI adoption:
- High volume of complex and unstructured data: Pharmacovigilance relies on various data sources, including spontaneous reporting systems, social media, electronic health records, and literature.
- Detection of rare adverse events: AI models often require significant data to learn effectively. However, detecting rare adverse drug reactions can be difficult as there may be insufficient data for training, leading to reduced accuracy in identifying less common safety signals.
- Bias in AI algorithms: AI tools can develop biases based on the data they are trained on. For instance, under-reporting certain demographics or geographical regions can skew the model's ability to use AI for signal detection in pharmacovigilance systems accurately for those populations.
- Ensuring timely signal detection and prioritization: The need to prioritize safety signals in real-time is critical. Balancing speed and accuracy for early detection while avoiding false positives or missed signals is a complex challenge.
- Regulatory and validation constraints for AI algorithms: Unlike other AI applications, implementing AI in pharmacovigilance for drug safety demands strict validation of algorithms to comply with safety and regulatory requirements. This involves ongoing model retraining, auditing, and validation to ensure any AI-driven safety assessments are reliable and compliant with evolving regulations.
The challenges listed above underscore the need for robust AI models in pharmacovigilance. These models must be tailored to handle the unique complexities of the field, including complex data, regulatory constraints, and timely signal detection in drug safety workflows.
Regulatory perspectives on AI-enhanced pharmacovigilance
Regulatory bodies globally are recognizing the growing role of AI in pharmacovigilance and are working on adapting guidelines to facilitate its safe and effective use. In this context, professionals in the pharmaceutical and regulatory sectors, including compliance officers and researchers, play a crucial role. Here are three key points:
- Adaptation of existing regulations: Authorities like the FDA and EMA are updating guidelines to include standards for implementing AI in pharmacovigilance for drug safety, ensuring technologies align with established safety protocols.
- Emphasis on compliance and transparency: Regulators stress the need for transparency in AI algorithms and processes, requiring companies to provide documentation and validation for AI-driven decisions to maintain public trust and ensure compliance when automating the pharmacovigilance process.
- Global harmonization of standards: Ensuring AI tools meet international safety regulations is critical. Compliance with standards like GVP (Good Pharmacovigilance Practices) helps guarantee consistent and effective use of AI for signal detection in pharmacovigilance systems across different regions and markets.
How AI enhances collaboration between stakeholders
AI improves collaboration among key stakeholders in pharmacovigilance by simplifying data sharing and communication. This brings pharmaceutical companies, regulators, healthcare providers, and patients closer together for better drug safety.
- Pharmaceutical companies and regulators: AI tools offer faster, transparent data analysis, helping both sides stay aligned on safety issues and compliance needs.
- Healthcare providers and patients: AI-powered monitoring of adverse reactions gives healthcare professionals the insights they need to make informed decisions, while patients benefit from quicker and more accurate safety reporting.
- Better communication and coordination: With AI, sharing safety information is seamless and secure, allowing for faster signal detection and coordinated risk assessments.
If you want to enhance collaboration and improve your pharmacovigilance processes, Binariks is here to help! Check out our AI development services and see how we can bring efficiency and clarity to your drug safety workflows.
Share

